The impact of CD remission on clinical picture and hypercortisolism-related comorbidities is still controversial. The current knowledge suggests that long-term CD surgical remission is associated with increased metabolic and vascular damage, not only if compared to active disease, but also even after long-term normalization of cortisol secretion [17]. If CD recurs after successful TSS, or if surgery fails/is not feasible, cortisol excess can be treated with medical therapy. Likewise, long-term studies (> 2 years) on the clinical effects of medical therapy on CD are lacking. Some prospective registry studies have been published [1], only one retrospective study on long-term use of ketoconazole described a multicentric cohort of CD patients without a control group [18].
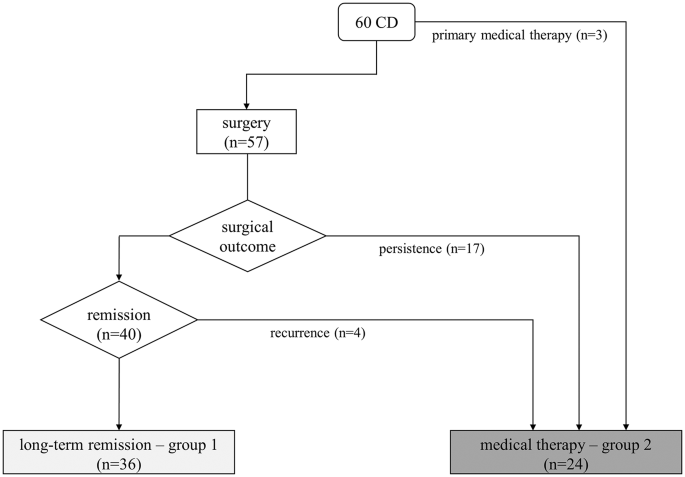
In our study, we enrolled 60 patients with CD diagnosed and treated in a single tertiary care center, with sustained and long-term (2 and 5 years) UFC normalization after surgery or during medical therapy. As expected, UFC levels at baseline were different in the two groups, due to the distinct starting point of medical history: a patient with persistent-recurrent CD after pituitary surgery presents with lower UFC than the new diagnosis. After surgical remission, patients achieved the recovery of salivary cortisol rhythm and the complete suppression of cortisol after 1-mg DST (investigated after substitutive glucocorticoid treatment discontinuation) in almost all cases. On the contrary, if eucortisolism is achieved with long-term medical therapy the recovery of salivary cortisol rhythm was observed only in half of patients and only few of them showed cortisol suppression after 1-mg DST within the 5 years observation time. Patients who were more resistant to the recovery of cortisol rhythm were more likely to receive combined treatment, even if no treatment is superior to others in normalizing salivary cortisol rhythm, in line with previous reports [1, 18, 19].
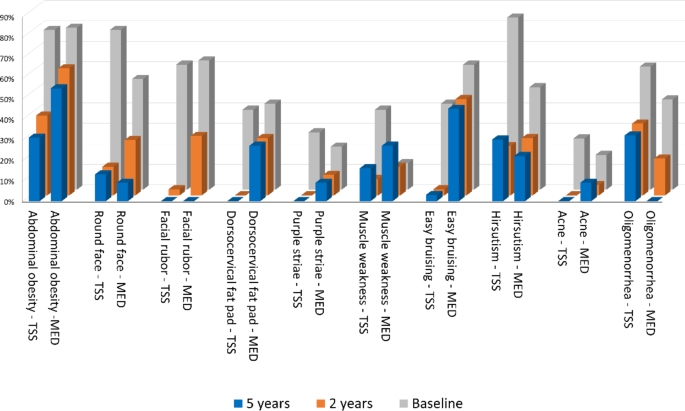
Within 2 years, patients in the surgical remission group showed a marked improvement of all phenotypic traits common at CD diagnosis compared to those in medical therapy. As observed also in other series of CD patients in remission [20], abdominal obesity persisted more than other clinical features over time, leading to an impaired body composition especially in the medically treated group [21]. Considering hyperandrogenism, acne improvement was more relevant at 2 and 5-years of follow up, probably due to a differential effect of ACTH-dependent adrenal androgens compared to hirsutism.
The impaired cortisol rhythm was a predictor of the long-lasting of most CD phenotypic features, as round face, buffalo hump, facial rubor, abdominal obesity, proximal myopathy and bruisability. A more severe clinical phenotype at baseline can explain a reduced control of hypercortisolism in monotherapy, requiring drug combination, and signs or symptoms are likely to persist despite the normalization of UFC [22]. In this study, no medication outperformed the others in terms of recovery from the CD phenotype.
The aetiology of hypertension and dyslipidemia is known to be heterogeneous, since both are influenced also by age at diagnosis and BMI, causing low rates of remission after UFC normalization [23, 24]. Arterial hypertension showed a decreasing trend with the best response within 2 years after UFC normalization only after surgical remission. Patients with disrupted salivary cortisol rhythm were more likely to remain hypertensive during the 5 years follow-up. Likewise, DM persistence during follow up correlates to impaired salivary cortisol rhythm and not with UFC. This finding is in contrast with the observations of Schernthaner-Reiter et al. [25]. on CD remission, and, on the contrary, supports data described by Guarnotta et al. [22]. Newell-Price et al.. recently found that when UFC and LSNC are both normal in patients treated with pasireotide, the rise in HbA1c levels is less evident than in patients with normal UFC but uncontrolled LNSC [26]. This observation underlines the importance of the impaired cortisol rhythm in the glucose impairment pathogenesis in CD. During the 5 years observation time, a worsening of previously diagnosed cardiovascular conditions, or novel acute vascular events, was not observed in both groups. This finding suggested that normalized UFC and intensive treatment of cardio-metabolic CD comorbidities play a fundamental role in reducing cardiovascular mortality [27]. A minor impact of CD therapy was observed in dyslipidemia, which persisted in both groups, with minimal improvement over time (−22% in surgical and − 6% in medical cohort). The criterion of 100 mg/dL LDL cut-off identifies a moderate CV risk reflecting the main focus of the study: the assessment of cardiometabolic complication after CD remission, assuming that they present a lower cardiovascular risk compared to patients with overt hypercortisolism.
Plasma hypercoagulability, with shortened aPTT, was found in all patients with active hypercortisolism. In the 5 years observation time, this parameter showed latency in increasing in both groups and in none achieved normality (> 28s). As previously observed in other studies, no correlation is observed between aPTT and any of the explored hormonal parameters [22, 28]. At 2- and 5 years, instead, shorter aPTT was observed during medical treatment than after surgical remission cohort. In both groups a shorter aPTT was associated with bruisability, which is related to impaired LNSC, strengthening the role of the impaired cortisol rhythm as a major driver of hypercoagulability. Also, Ferrante et al.. observed the long latency of plasma hypercoagulability, persisting for years after biochemical remission of CD: in that series thrombophilia appeared to be reversible within 5 years [29], while in our cohort the recovery takes longer.
Additionally, sexual differences characterize patients with patients with Cushing’s syndrome and hypogonadism in hypercortisolism is known to further increase the cardiovascular risk [30, 31]. However, it was not an interfering factor in our study population since hypopituitarism was considered an exclusion criterion, no case of new-onset hypogonadism was reported (even in male patients treated with ketoconazole), and the menopause transition in six women during the observation was not considered relevant.
The limits of the present study are its retrospective design, the variability of concomitant treatments, the heterogenous combinations of medical therapy used in clinical practice, the presence of treatment-specific adverse events that mimic the effects of hypercortisolism (such as pasireotide-induced DM and hypertension with metyrapone), the unpredictable effect of previous treatments, including radiotherapy. We considered UFC and LNSC as markers of hypercortisolism remission; nonetheless we acknowledge that both of them present some limitations, especially during medical treatment. The former considers the whole cortisol secretion during the day, and albeit UFC normalization is the main outcome of all trials for medical treatment [32, 33] it does not detect mild hypercortisolism. On the other hand, a normal LNSC does not fully reflect a normal circadian rhythm: only high cortisol levels in the morning with a decline in the night are able to restore clock-related activities [34].
Its strengths are the complete patient characterization in a single tertiary care center, the comparative study design, and the standardized protocols for diagnosis and long-term follow-up. In particular, samples have been processed within a single laboratory with accurate methods (LC-MS for urinary and salivary steroids), and all endocrine aspects of hypercortisolism were considered (overall daily cortisol production by UFC, circadian cortisol rhythm, and the recovery of the hypothalamic-pituitary axis by 1-mg DST overnight test).
To conclude, despite UFC normalization in both groups during follow-up, surgical remission results in more rapid and relevant improvements in CD phenotype and comorbidities. During medical therapy the UFC levels can be higher than after surgery, although in the normal range, and the normalization of LNSC is not always achieved: both conditions suggests that stricter criteria should be considered to define eucortisolism in patients with CD under medical treatment. Conditions such as obesity, hypertension, dyslipidemia, and hypercoagulability are not completely reversible in a 5-year observation time even in the surgical remission group. This observation underlines that all the comorbidities, independently of the normalization of UFC, must be intensively treated. Moreover, UFC normalization should not be considered the only biochemical goal to be reached, since the persistence of comorbidities seems to be more related to an impaired cortisol rhythm rather than to the cortisol secretory burden.