Abstract
Cushing’s disease; obesity; growth retardation; osteoporosis
1. Introduction
2. Case Presentation

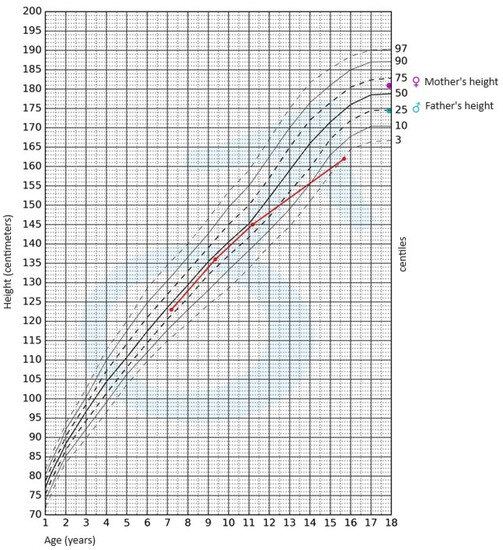
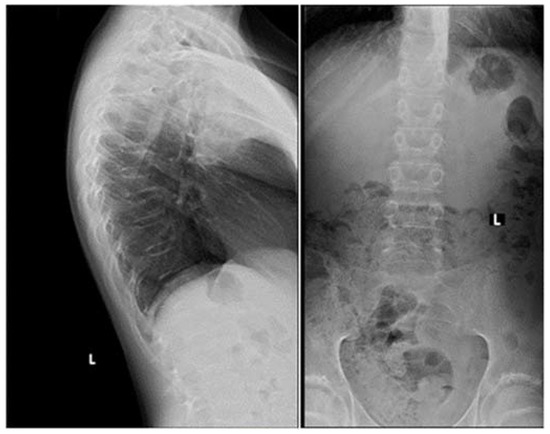
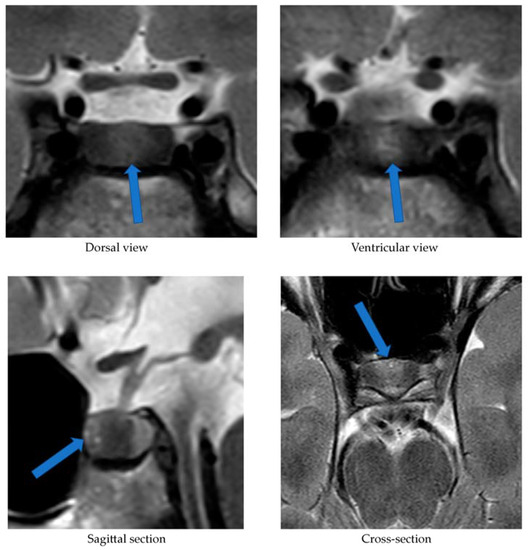
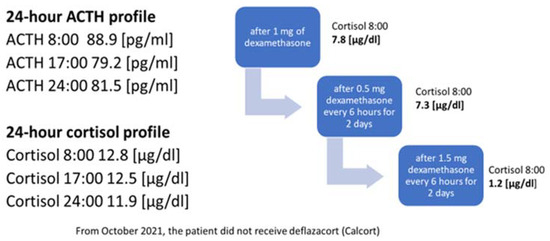

3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrigno, R.; Hasenmajer, V.; Caiulo, S.; Minnetti, M.; Mazzotta, P.; Storr, H.L.; Isidori, A.M.; Grossman, A.B.; De Martino, M.C.; Savage, M.O. Paediatric Cushing’s disease: Epidemiology, pathogenesis, clinical management and outcome. Rev. Endocr. Metab. Disord. 2021, 22, 817–835. [Google Scholar] [CrossRef]
- Stratakis, A. Cushing syndrome in pediatrics. Endocrinol. Metab. Clin. N. Am. 2012, 41, 793–803. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Hull, B.; Tyrawa, K.; Kalicka-Kasperczyk, A.; Zieliński, G.; Starzyk, J. Cushing disease in children and adolescents—Assessment of the clinical course, diagnostic process, and effects of the treatment—Experience from a single paediatric centre. Pediatr. Endocrinol. Diabet. Metab. 2019, 25, 127–143. [Google Scholar] [CrossRef]
- Concepción-Zavaleta, M.J.; Armas, C.D.; Quiroz-Aldave, J.E.; García-Villasante, E.J.; Gariza-Solano, A.C.; Del Carmen Durand-Vásquez, M.; Concepción-Urteaga, L.A.; Zavaleta-Gutiérre, F.E. Cushing disease in pediatrics: An update. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 87–97. [Google Scholar] [CrossRef]
- Detomas, M.; Ritzel, K.; Nasi-Kordhishti, I.; Wolfsberger, S.; Quinkler, M.; Losa, M.; Tröger, V.; Kroiss, M.; Fassnacht, M.; Vila, G.; et al. Outcome of CRH stimulation test and overnight 8 mg dexamethasone suppression test in 469 patients with ACTH-dependent Cushing’s syndrome. Front. Endocrinol. 2022, 13, 955945. [Google Scholar] [CrossRef] [PubMed]
- Lodish, M.B.; Hsiao, H.P.; Serbis, A.; Sinaii, N.; Rothenbuhler, A.; Keil, M.F.; Boikos, S.A.; Reynolds, J.C.; Stratakis, C.A. Effects of Cushing disease on bone mineral density in a pediatric population. J. Pediatr. 2010, 56, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.; Colao, A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4 (Suppl. S7), 611–629. [Google Scholar] [CrossRef] [PubMed]
- Lonser, R.R.; Wind, J.J.; Nieman, L.K.; Weil, R.J.; DeVroom, H.L.; Oldfield, E.H. Outcome of surgical treatment of 200 children with Cushing’s disease. J. Clin. Endocrinol. Metab. 2013, 98, 892–901. [Google Scholar] [CrossRef]
- Detomas, M.; Deutschbein, T.; Tamburello, M.; Chifu, I.; Kimpel, O.; Sbiera, S.; Kroiss, M.; Fassnacht, M.; Altieri, B. Erythropoiesis in Cushing syndrome: Sex-related and subtype-specific differences. Results from a monocentric study. J. Endocrinol. Investig. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 2015, 1, 14. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health; potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Querini, P.R.; Giustina, A. Low vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J. Clin. Endocrinol. Metab. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- di Filippo, L.; Allora, A.; Doga, M.; Formenti, A.M.; Locatelli, M.; Rovere Querini, P.; Frara, S.; Giustina, A. Vitamin D levels are associated with blood glucose and BMI in COVID-19 patients, predicting disease severity. J. Clin. Endocrinol. Metab. 2022, 107, 348–360. [Google Scholar] [CrossRef]
- Storr, H.L.; Chan, L.F.; Grossman, A.B.; Savage, M.O. Pediatric Cushing’s syndrome: Epidemiology, investigation and therapeutic advances. Trends Endocrinol. Metab. 2007, 18, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Lee, J.; Kim, G.E.; Yeo, J.Y.; Kim, S.H.; Nam, M.; Kim, Y.S.; Hong, S. Case of Cushing syndrome diagnosed by recurrent pathologic fractures in a young woman. J. Bone Metab. 2012, 19 (Suppl. S2), 153–158. [Google Scholar] [CrossRef] [PubMed]
- Elenius, H.; McGlotten, R.; Nieman, L.K. Ovine CRH stimulation and 8 mg dexamethasone suppression tests in 323 patients with ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. S5), 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.O.; Storr, H.L. Pediatric Cushing’s disease: Management issues. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S2), 171–175. [Google Scholar] [CrossRef]
- Detomas, M.; Ritzel, K.; Nasi-Kordhishti, I.; Schernthaner-Reiter, M.H.; Losa, M.; Tröger, V.; Altieri, B.; Kroiss, M.; Kickuth, R.; Fassnacht, M.; et al. Bilateral inferior petrosal sinus sampling with human CRH stimulation in ACTH-dependent Cushing’s syndrome: Results from a retrospective multicenter study. Eur. J. Endocrinol. 2023, 188 (Suppl. S5), 448–456. [Google Scholar] [CrossRef]
- Chen, S.; Chen, K.; Wang, S.; Zhu, H.; Lu, L.; Zhang, X.; Tong, A.; Pan, H.; Wang, R.; Lu, Z. The optimal cut-off of BIPSS in differential diagnosis of ACTH-dependent Cushing’s syndrome: Is stimulation necessary? J. Clin. Endocrinol. Metab. 2020, 105 (Suppl. S4), 1673–1685. [Google Scholar] [CrossRef]
- Eviz, E.; Yesiltepe, M.G.; Arduc, A.A.; Erbey, F.; Guran, T.; Hatun, S. An overlooked manifestation of hypercortisolism—Cerebral cortical atrophy and challenges in identifying the etiology of hypercortisolism: A report of 2 pediatric cases. Horm. Res. Paediatr. 2023, 27. [Google Scholar] [CrossRef]
- Paragliola, R.M.; Corsello, A.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Cushing’s syndrome effects on the thyroid. Int. J. Mol. Sci. 2021, 22, 3131. [Google Scholar] [CrossRef]
- Pomahacova, R.; Paterova, P.; Nykodymova, E.; Sykora, J.; Krsek, M. Pediatric Cushing’s disease: Case reports and retrospective review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 2022, 166. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
Share and Cite
Łupińska, A.; Aszkiełowicz, S.; Zieliński, G.; Stawerska, R.; Lewiński, A. Osteoporosis as the First Sign of Cushing’s Disease in a Thin 16-Year-Old Boy—A Case Report. J. Clin. Med. 2023, 12, 5967. https://doi.org/10.3390/jcm12185967
Chicago/Turabian StyleŁupińska, Anna, Sara Aszkiełowicz, Grzegorz Zieliński, Renata Stawerska, and Andrzej Lewiński. 2023. “Osteoporosis as the First Sign of Cushing’s Disease in a Thin 16-Year-Old Boy—A Case Report” Journal of Clinical Medicine 12, no. 18: 5967. https://doi.org/10.3390/jcm12185967
Article Metrics
Citations
Article Access Statistics
For more information on the journal statistics, click here.
Filed under: Cushing's, Diagnostic Testing, growth hormone, symptoms | Tagged: Cushing's Disease, Growth Hormone Deficiency, osteoporosis, Teen | Leave a comment »



