JCEM Case Reports, Volume 2, Issue 11, November 2024, luae183, https://doi.org/10.1210/jcemcr/luae183
Abstract
Secondary adrenal insufficiency is a life-threatening condition that may arise in the setting of iatrogenic Cushing syndrome. Intra-articular corticosteroid injections (IACs) are a standard treatment for osteoarthritis, and they carry a high risk of secondary central adrenal suppression (SAI). We present the case of a 43-year-old woman who was referred to reproductive endocrinology for evaluation of abnormal uterine bleeding with a provisional diagnosis of perimenopause. She reported new-onset type 2 diabetes mellitus, abdominal striae, hot flashes, and irregular menses. Laboratory evaluation revealed iatrogenic Cushing syndrome and SAI attributable to prolonged use of therapeutic IACs for osteoarthritis. Treatment included hydrocortisone replacement and discontinuation of IACs followed by hydrocortisone taper over the following 16 months that resulted in the return of endogenous ovarian and adrenal function. This case demonstrates the many hazards of prolonged IAC use, including suppression of ovarian and adrenal function and iatrogenic SAI.
Introduction
Intra-articular corticosteroid injections (IACs) are commonly used for the treatment of symptomatic osteoarthritis [1]. Synovial injections carry the highest risk of secondary central adrenal suppression (SAI) [2-5]. Further, exogenous glucocorticoid administration may also result in secondary Cushing syndrome. Symptoms associated with exogenous glucocorticoid administration vary significantly, and misdiagnosis is common [6, 7]. Here, we present a case of exogenous IAC use resulting in SAI and Cushing syndrome in a 43-year-old woman who was referred for evaluation and treatment of abnormal uterine bleeding with a provisional diagnosis of perimenopause.
Case Presentation
A 43-year-old woman with a past medical history of fibromyalgia, osteoarthritis, bursitis, asthma, gastroesophageal reflux, and diabetes was referred to reproductive endocrinology with a chief complaint of hot flashes for over 2 years and a presumptive diagnosis of perimenopause. Approximately 2 years before the onset of her symptoms, she reported irregular menses, followed by 11 months of amenorrhea, then 3 menstrual intervals with prolonged bleeding lasting 45, 34, and 65 days, respectively. She reported menarche at 11 years old, regular menstrual cycles until the last 2 years, and 4 pregnancies that were spontaneously conceived. She delivered 3 liveborn term children and had one spontaneous miscarriage. Her only complication of pregnancy was gestational hypertension during her last pregnancy that occurred 9 years prior when she was 34 years old.
In addition to menstrual irregularity, she also reported hot flashes, increasing truncal weight gain over the last 5 years, new-onset diabetes mellitus, and hypertension. Eighteen months prior to referral, she had an endometrial biopsy, which demonstrated secretory endometrium without hyperplasia, and cervical cancer screening was negative.
She initially reported the following medications: inhaled fluticasone/propionate + salmeterol 232 mcg + 14 mcg as needed and albuterol 108 mcg as needed. Her daily medications were glimepiride 1 mg, furosemide 20 mg, omeprazole 20 mg, montelukast 10 mg, azelastine hydrochloride 137 mcg, ertugliflozin 5 mg, and tiotropium bromide 2.5 mg. Importantly, she did not report IAC treatments.
Diagnostic Assessment
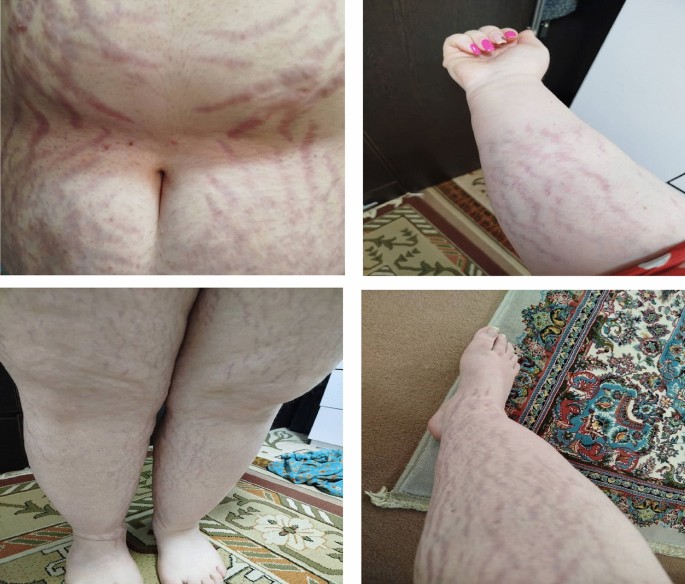
Initial physical examination showed height of 160 cm, weight of 103.4 kg, body mass index (BMI) of 46 kg/m2, and blood pressure (BP) of 128/80. Physical exam was significant for round facies with plethora, bilateral dorsocervical neck fat pads, and violaceous striae on her abdomen and upper arms (Fig. 1). The patient ambulated with a cane and reported severe bilateral proximal leg atrophy and weakness.

Figure 1.
Abdominal and upper extremity striae prior to treatment with truncal obesity immediately before (A) and 1 year after initial diagnosis (B).
A laboratory evaluation was recommended but was not initially completed. She was scheduled for a transvaginal ultrasound that required prior authorization; the pelvic ultrasound showed a heterogeneous and thickened anterior uterine wall, suggestive of adenomyosis, with a posterior intramural fibroid measuring 15 × 15 mm and an anterior intramural fibroid measuring 15 × 8 mm. Endometrial lining was thin at 5 mm. Both ovaries were small, without masses or antral follicles. Three-dimensional reconstruction showed a normal uterine cavity with some heterogeneity of the endometrial lining but no discrete masses suggestive of polyps or intracavitary fibroids as the cause of irregular bleeding. Upon additional questioning, she acknowledged receiving bilateral shoulder, hip, and knee injections of triamcinolone 80 mg every 2 to 3 months to each joint for about 5 years. Table 1 shows the initial laboratory evaluation and includes age-appropriate low ovarian reserve as evidenced by anti-Müllerian hormone (AMH), secondary hypothalamic hypogonadism, diabetes mellitus, and central adrenal suppression. Of note, the diabetes mellitus developed after 3 years of IAC use. Additional diagnostic assessment for adrenal insufficiency by synacthen testing was scheduled, however, the patient declined further investigation.
Initial laboratory values at presentation
|
Result |
Reference range |
| Basic metabolic panel |
| Sodium |
141 mEq/L; 141 mmol/L |
135 to 145 mEq/L; 135 to 145 mmol/L |
| Potassium |
3.7 mEq/L; 3.7 mmol/L |
3.7 to 5.2 mEq/L; 3.7 to 5.20 mmol/L |
| Chloride |
104 mEq/L; 104 mmol/L |
96 to 106 mEq/L; 96 to 106 mmol/L |
| Carbon dioxide |
25 mEq/L; 25 mmol/L |
23 to 29 mEq/L; 23 to 29 mmol/L |
| Creatinine |
0.42 mg/dL; 37.14 µmol/L |
0.6 to 1.3 mg/dL; 53 to 114.9 µmol/L |
| Urea nitrogen |
14 mg/dL; 5 mmol/L |
6 to 20 mg/dL; 2.14 to 7.14 mmol/L |
| Adrenal function |
| Cortisol |
0.8 µg/dL; 22.07 nmol/L |
4-22 µg/dL; 138-635 nmol/L |
| ACTH |
<5 pg/mL; <1 pmol/L |
6-50 pg/mL; 5.5-22 pmol/L |
| DHEAS |
8 mcg/dL; 0.02 µmol/L |
15-205 mcg/dL; 1.36-6.78 µmol/L |
| Endocrine function |
| HbA1c |
8.5% |
<5.7% |
| Random glucose |
124 mg/dL; 6.9 mmol/L |
80-100 mg/dL; 4.4-5.5 mmol/L |
| TSH |
1.74 mIU/L |
0.5-5 mIU/L |
| tT4 |
10.5 µg/dL; 135.2 nmol/L |
5.0-12.0 µg/dL; 57-148 nmol/L |
| Free T4 index |
2.6 ng/dL; 33.4 pmol/L |
0.7-1.9 ng/dL; 12-30 pmol/L |
| tT3 |
165 ng/dL; 2.5 nmol/L |
60-180 ng/dL; 0.9-2.8 nmol/L |
| TPO antibody |
Negative |
n/a |
| Ovarian function |
| FSH |
5.6 IU/L |
4.5-21.5 IU/L |
| LH |
2.9 IU/L |
5-25 IU/L |
| Progesterone |
<0.5 ng/mL; 1.6 nmol/L |
Varies |
| Estradiol |
21 pg/mL; 77.1 pmol/L |
Varies |
| AMH |
1.1 ng/mL; 7.9 pmol/L |
1.0-3.0 ng/mL; 2.15-48.91 pmol/L |
Abbreviations: ACTH, adrenocorticotropic hormone; AMH, anti-Müllerian hormone; DHEAS, dehydroepiandrosterone sulfate; eGFR, estimated glomerular filtration rate; FSH, follicle-stimulating hormone; HbA1c, hemoglobin A1C; LH, luteinizing hormone; TPO antibody, thyroid peroxidase antibody; TSH, thyroid stimulating hormone; tT4, total thyroxine.
Treatment
The patient was immediately started on hydrocortisone 10 mg twice daily for glucocorticoid replacement, which was gradually reduced to 5 mg daily each morning at 16 months. Endocrine function testing was trended over the following months as replacement cortisone therapy was tapered.
Outcome and Follow-Up
Within 6 months of replacement and cessation of IACs, hot flashes ceased, and she reported regular menses. She lost 6.8 kg, her truncal obesity and striae significantly improved (Fig. 1), and she could now ambulate without assistance. Her glycated hemoglobin (HbA1c) level decreased from 8.5% to 6.8%. Fourteen months after her initial diagnosis and cessation of IAC, laboratory studies demonstrated partial recovery of adrenal and ovarian function and improved metabolism, as evidenced by increases in morning cortisol, adrenocorticotropic hormone (ACTH), and dehydroepiandrosterone sulfate (DHEAS), and decreased HbA1c. At 16 months, she had a return of ovulatory ovarian function.
Discussion
Cortisol is the main glucocorticoid secreted by human adrenal glands. The secretion pattern is precisely regulated by an integrated limbic-hypothalamic-pituitary (LHP) drive with the physiologic goal of homeostasis [1]. Conditions that result in deviations in glucocorticoid concentrations carry a variety of consequences. Our patient was referred because of a provisional diagnosis of abnormal uterine bleeding and perimenopause, which distracted from recognition of iatrogenic Cushing syndrome and secondary central adrenal suppression. This patient vignette underscores the importance of explicitly asking patients about nonoral medications, as patients may not report their use.
Exogenous administration of long-acting synthetic glucocorticoids may suppress adrenal function via negative feedback at the limbic and hypothalamic levels, which was reflected in this patient by undetectable ACTH and low cortisol levels (Table 1). In addition, excess glucocorticoid levels result in other neuroendocrine concomitants, including suppression of gonadotropin-releasing hormone (GnRH) drive that results in hypothalamic hypogonadism [8, 9], decreased luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels, and anovulation despite AMH levels indicating residual ovarian reserve [10]. The clinical phenotype is variable and reflects individual glucocorticoid receptor sensitivities [9].
Regardless of cause, Cushing syndrome often presents with hallmark features of central obesity, violaceous striae, easy bruising, round facies, and nuchal adiposity with lower limb muscle atrophy and loss of strength [11]. Additionally, glucocorticoid excess causes insulin resistance and metabolic syndrome [8]. Truncal obesity is a common presenting symptom of excess cortisol. Cortisol inhibits metabolic response to insulin centrally and peripherally and increases gluconeogenesis, which together predispose to and cause diabetes [10].
Exogenous use of injectable glucocorticoids carries the highest risk of adrenal suppression when compared to other routes of exogenous steroids [5]. Patients typically report fatigue, malaise, and gastrointestinal complaints. Oligomenorrhea is a common presenting complaint in women, as was the case in our patient. Hyponatremia, water retention, and hypotension may occur in SAI because of endogenous glucocorticoid deficiency. A thorough laboratory evaluation in this patient revealed low LH, FSH, estradiol, and progesterone levels, indicating hypothalamic hypogonadism and not perimenopause/menopause [12] and low levels of cortisol, ACTH, and DHEAS confirmed SIA [10].
Adrenal insufficiency can be a life-threatening condition that requires supplementation with glucocorticoids [10, 13, 14]. A review of patients diagnosed with SAI suggested tapering of hydrocortisone before discontinuing all replacement therapy and revealed most patients recover without the need for exogenous steroids after 2 years from diagnosis [14]. ACTH stimulation testing may indicate the return of adrenal function [14, 15]. Our patient showed increased ACTH, cortisol, and DHEAS at 14 months. Ovulatory ovarian function, indicated by progesterone < 5 ng/mL (< 1.59 nmol/L) (Table 2), returned at 16 months after cessation of IACs. The improvement in adrenal and ovarian function following cessation of IACs and tapering of hydrocortisone replacement therapy was accompanied by decreased HbA1c, weight loss, truncal obesity, and stria, and increased muscle strength scalp hair.
Table 2.
Endocrine lab results 7 years prior, at presentation (T0), and over the next 16 months
| Analyte |
Reference range |
7 years prior |
T0 |
1 month |
7 months |
13 months |
14 months |
16 months |
| DHEAS |
15-205 µg/dL; 1.36-6.78 nmol/L |
— |
8 µg/dL; 0.22 nmol/L |
5 µg/dL;
0.14 nmol/L |
6 µg/dL;
0.16 nmol/L |
22 µg/dL; 0.59 nmol/L |
28 µg/dL; 0.76 nmol/L |
24 µg/dL; 0.65 nmol/L |
| Cortisol |
4-22 µg/dL; 138-635 nmol/L |
— |
0.9 µg/dL;
24.83 nmol/L |
5.8 µg/dL;
160.01 nmol/L |
3.0 µg/dL;
82.76 nmol/L |
3.9 µg/dL;
107.59 nmol/L |
11.2 µg/dL;
308.99 nmol/L |
12.6 µg/dL;
347.61 nmol/L |
| ACTH |
6-50 pg/mL; 5.5-22 pmol/L |
— |
<5 pg/mL;<1.10 pmol/L |
<5 pg/mL;<1.10 pmol/L |
<5 pg/mL;<1.10 pmol/L |
<5 pg/mL;<1.10 pmol/L |
11 pg/mL;
2.42 pmol/L |
10 pg/mL;
2.20 pmol/L |
| HbA1c |
<5.7% |
5.0% |
8.5% |
8.5% |
7.8% |
5.8% |
5.7% |
5.7% |
| LH |
5-25 IU/L |
— |
5.8 IU/L |
2.9 IU/L |
— |
3.3 IU/L |
5.2 IU/L |
5.7 IU/L |
| FSH |
4.5-21.5 IU/L |
— |
6.2 IU/L |
5.6 IU/L |
— |
2.0 IU/L |
3.5 IU/L |
1.3 IU/L |
| Estradiol |
Varies |
— |
21 pg/mL;
77.09 pmol/L |
74 pg/mL;
271.65 pmol/L |
— |
101 pg/mL;
370.77 pmol/L |
— |
121 pg/mL;
444.19 pmol/L |
| Progesterone |
Varies |
— |
<0.5 ng/mL;<1.59 nmol/L |
<0.5 ng/mL;<1.59 nmol/L |
— |
<0.5 ng/mL;<1.59 nmol/L |
— |
6.6 ng/mL;
20.99 nmol/L |
Abbreviations: ACTH, adrenocorticotropic hormone, DHEAS, dehydroepiandrosterone sulfate, FSH, follicle-stimulating hormone, LH, luteinizing hormone, T0, time at presentation.
In conclusion, exogenous glucocorticoids, specifically intra-articular injections, carry the highest potential for iatrogenic Cushing syndrome and secondary adrenal insufficiency. Glucocorticoid excess has a variable presentation that often obscures diagnosis. As this scenario demonstrates, careful physical and laboratory assessment and tapering of hydrocortisone replacement eventually can lead to restoration of adrenal, ovarian, and metabolic function and improved associated symptoms.
Learning Points
- Exogenous intra-articular glucocorticoid use may suppress adrenal and ovarian function via central suppression of ACTH and GnRH.
- Cushing syndrome presents with a broad spectrum of signs and symptoms that may be mistaken for individual conditions, such as perimenopause and isolated diabetes mellitus.
- Exogenous steroid use may lead to Cushing syndrome and subsequent adrenal insufficiency, which is life-threatening.
- Treatment of adrenal insufficiency with a long-term exogenous glucocorticoid taper allows for subsequent return of adrenal and ovarian function.
Contributors
All authors contributed to authorship. S.L.B. was involved in the diagnosis and management of the patient, and manuscript editing. S.A. was involved in patient follow-up and manuscript development. J.M.G. was responsible for manuscript development and editing. All authors reviewed and approved the final draft.
Funding
None declared.
Disclosures
S.L.B. reports ClearBlue Medical Advisory Board, 2019—present
Emblem Medical Advisory Board, Amazon Services, 2022—present
Medscape, 2023
Myovant, May 2023
Omnicuris, 2023
Sage Therapeutics and Biogen Global Medical, Zuranolone OB/GYN Providers Advisory Board, Dec 2022, March 2023
Member, Board of Trustees, Salem Academy and College, Salem, NC: 2018-present (Gratis)
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Originally data generated and analyzed in this case are reported and included in this article.
References
1
Johnston
PC
,
Lansang
MC
,
Chatterjee
S
,
Kennedy
L
.
Intra-articular glucocorticoid injections and their effect on hypothalamic-pituitary-adrenal (HPA)-axis function
.
Endocrine
.
2015
;
48
(
2
):
410
‐
416
.
2
Stout
A
,
Friedly
J
,
Standaert
CJ
.
Systemic absorption and side effects of locally injected glucocorticoids
.
PM R
.
2019
;
11
(
4
):
409
‐
419
.
3
Prete
A
,
Bancos
I
.
Glucocorticoid induced adrenal insufficiency
.
BMJ
.
2021
;
374
:
n1380
.
4
Herman
JP
,
McKlveen
JM
,
Ghosal
S
, et al.
Regulation of the hypothalamic-pituitary-adrenocortical stress response
.
Compr Physiol
.
2016
;
6
(
2
):
603
‐
621
.
5
Broersen
LH
,
Pereira
AM
,
Jørgensen
JO
,
Dekkers
OM
.
Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis
.
J Clin Endocrinol Metab
.
2015
;
100
(
6
):
2171
‐
2180
.
6
Tan
JW
,
Majumdar
SK
.
Development and resolution of secondary adrenal insufficiency after an intra-articular steroid injection
.
Case Rep Endocrinol
.
2022
;
2022
:
4798466
.
7
Colpitts
L
,
Murray
TB
,
Tahhan
SG
,
Boggs
JP
.
Iatrogenic cushing syndrome in a 47-year-old HIV-positive woman on ritonavir and inhaled budesonide
.
J Int Assoc Provid AIDS Care
.
2017
;
16
(
6
):
531
‐
534
.
8
Lee
SM
,
Hahm
JR
,
Jung
TS
, et al.
A case of Cushing’s syndrome presenting as endometrial hyperplasia
.
Korean J Intern Med
.
2008
;
23
(
1
):
49
‐
52
.
9
Yesiladali
M
,
Yazici
MGK
,
Attar
E
,
Kelestimur
F
.
Differentiating polycystic ovary syndrome from adrenal disorders
.
Diagnostics (Basel)
.
2022
;
12
(
9
):
2045
.
10
Raff
H
,
Sharma
ST
,
Nieman
LK
.
Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia
.
Compr Physiol
.
2014
;
4
(
2
):
739
‐
769
.
11
Unuane
D
,
Tournaye
H
,
Velkeniers
B
,
Poppe
K
.
Endocrine disorders & female infertility
.
Best Pract Res Clin Endocrinol Metab
.
2011
;
25
(
6
):
861
‐
873
.
12
Peacock
K
,
Carlson
K
,
Ketvertis
KM.
Menopause.
StatPearls
.
StatPearls Publishing, Copyright © 2024, StatPearls Publishing LLC.
,
2024
.
13
Foisy
MM
,
Yakiwchuk
EM
,
Chiu
I
,
Singh
AE
.
Adrenal suppression and Cushing’s syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature
.
HIV Med
.
2008
;
9
(
6
):
389
‐
396
.
14
Draoui
N
,
Alla
A
,
Derkaoui
N
, et al.
Assessing recovery of adrenal function in glucocorticoid-treated patients: our strategy for screening and management
.
Ann Med Surg (Lond)
.
2022
;
78
:
103710
.
15
Joseph
RM
,
Hunter
AL
,
Ray
DW
,
Dixon
WG
.
Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review
.
Semin Arthritis Rheum
.
2016
;
46
(
1
):
133
‐
141
.
Abbreviations
-
ACTH
adrenocorticotropic hormone
-
AMH
-
DHEAS
dehydroepiandrosterone sulfate
-
FSH
follicle-stimulating hormone
-
HbA1c
-
IAC
intra-articular corticosteroid
-
LH
-
SAI
secondary central adrenal suppression
Published by Oxford University Press on behalf of the Endocrine Society 2024.
This work is written by (a) US Government employee(s) and is in the public domain in the US. See the journal About page for additional terms.
Filed under: adrenal, adrenal crisis, Cushing's, Rare Diseases, symptoms | Tagged: adrenal, adrenal insufficiency, Cushing's Syndrome, diabetes mellitus, perimenopause, synacthen testing | Leave a comment »