This is kind of a “cheat” post since it’s a compilation of other posts, web pages, message board posts and some original thoughts.
For all of my early life, I was the good, compliant, patient. I took whatever pills the doctor prescribed, did whatever tests h/she (most always a he) wrote for. Believed that whatever he said was the absolute truth. He had been to med school. He knew what was wrong with me even though he didn’t live in my body 24/7 and experience what I did.
I know a lot of people are still like this. Their doctor is like a god to them. He can do no wrong – even if they don’t feel any better after treatment, even if they feel worse. “But the doctor said…”
Anyway, I digress.
All this changed for me in 1983.
At first I noticed I’d stopped having my periods and, of course, I thought I was pregnant. I went to my Gynecologist who had no explanation. Lots of women lose their periods for a variety of reasons so no one thought that this was really significant.
Then I got really tired, overly tired. I would take my son to a half hour Choir rehearsal and could not stay awake for the whole time. I would lie down in the back of the van, set an alarm and sleep for the 30 minutes.
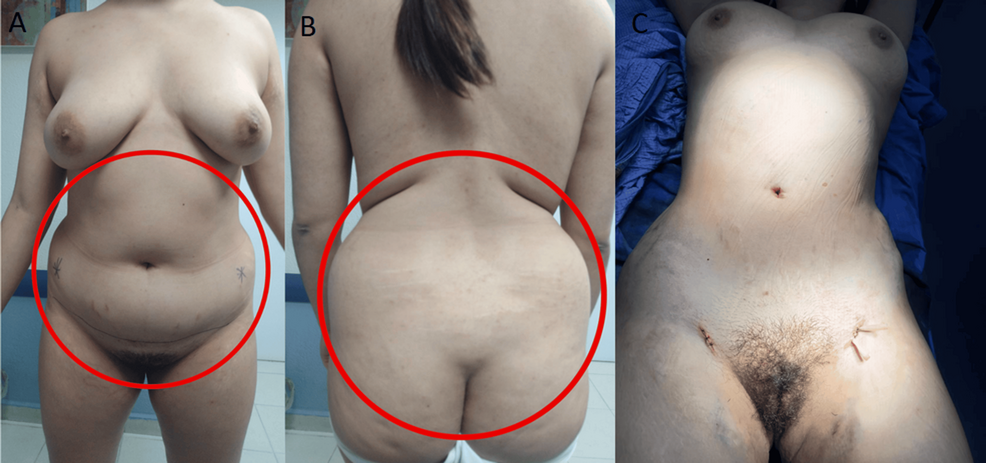
A whole raft of other symptoms started appearing – I grew a beard (Hirsuitism), gained weight even though I was on Weight Watchers and working out at the gym nearly every day, lost my period, everything hurt, got what is called a “moon face” and a “buffalo hump” on the back of my neck. I also got stretch marks. I was very depressed but it’s hard to say if that was because of the hormone imbalance or because I felt so bad and no one would listen to me.
I came across a little article in the Ladies Home Journal magazine which said “If you have these symptoms…ask your doctor about Cushing’s”. After that, I started reading everything I could on Cushing’s and asking my doctors. Due to all my reading at the library and medical books I bought, I was sure I had Cushing’s but no one would believe me. Doctors would say that Cushing’s Disease is too rare, that I was making this up and that I couldn’t have it.
I asked doctors for three years – PCP, gynecologist, neurologist, podiatrist – all said the now-famous refrain. It’s too rare. You couldn’t have Cushing’s. I kept persisting in my reading, making copies of library texts even when I didn’t understand them, keeping notes. I just knew that someone, somewhere would “discover” that I had Cushing’s.
My husband was on the doctors’ sides. He was sure it was all in my mind (as opposed to all in my head!) and he told me to just think “happy thoughts” and it would all go away.
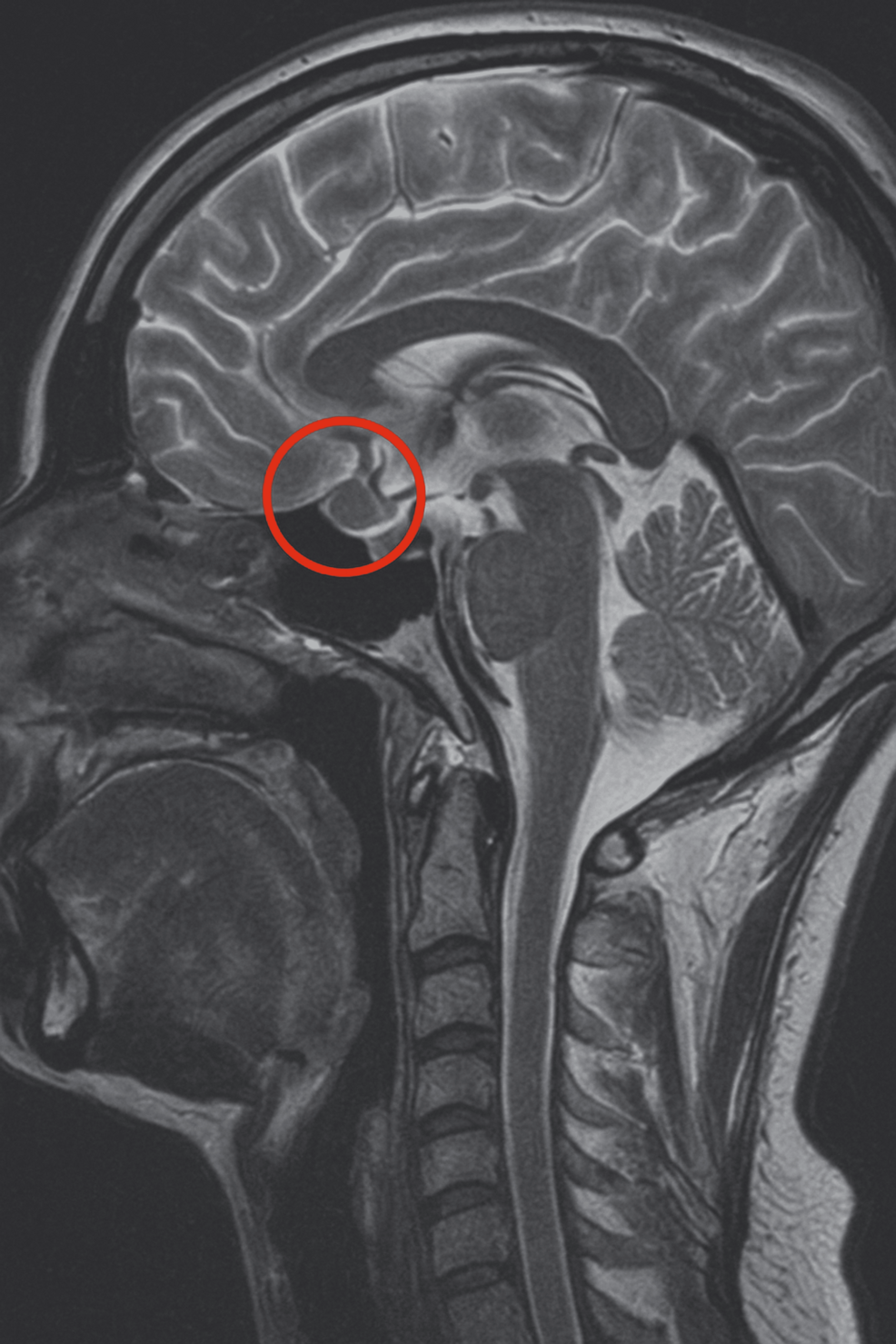
A Neurologist gave me Xanax. Since he couldn’t see my tumor with his Magnetic Resonance Imaging (MRI) machine there was “no possibility” that it existed. Boy was he wrong!
Later in 1986 I started bruising incredibly easily. I could touch my skin and get a bruise. On New Year’s Day of 1987 I started bleeding under the skin. My husband made circles around the outside perimeter each hour with a marker, like the rings of a tree. When I went to my Internist the next day he was shocked at the size. He now thought I had a blood disorder so he sent me to a Hematologist/Oncologist.
Fortunately, the Hematologist/Oncologist ran a twenty-four hour urine test and really looked at me. Both he and his partner recognized that I had Cushing’s. Of course, he was sure that he did the diagnosis. No matter that I had been pursuing this with other doctors for 3 years.
It was not yet determined if it was Cushing’s Disease (Pituitary) or Syndrome (Adrenal). However, he couldn’t help me any further so the Hematologist referred me to an Endocrinologist.
The Endocrinologist, of course, didn’t trust the other tests I had had done so I was back to square one. He ran his own multitude of tests. He had to draw blood at certain times like 9 AM. and 5 PM. There was a dexamethasone suppression test where I took a pill at 10 p.m. and gave blood at 9 am the next day. I collected gallons of urine in BIG boxes (Fun in the fridge!). Those were from 6 a.m. to 6 a.m. to be delivered to his office by 9 a.m. same day. I was always worried that I’d be stopped in rush hour and the police would ask about what was in that big container. I think I did those for a week. He also did standard neurological tests and asked lots of questions.
When the endo confirmed that I had Cushing’s in 1987 he sent me to a local hospital where they repeated all those same tests for another week and decided that it was not my adrenal gland (Cushing’s Syndrome) creating the problem. The doctors and nurses had no idea what to do with me, so they put me on the brain cancer ward.
When I left this hospital after a week, we didn’t know any more than we had before.
As luck would have it, NIH (National Institutes of Health, Bethesda, Maryland) was doing a clinical trial of Cushing’s. I live in the same area as NIH so it was not too inconvenient but very scary at first to think of being tested there. At that time I only had a choice of NIH, Mayo Clinic and a place in Quebec to do this then-rare pituitary surgery called a Transsphenoidal Resection. I chose NIH – closest and free. After I was interviewed by the Doctors there, I got a letter that I had been accepted into the clinical trial. The first time I was there was for 6 weeks as an inpatient. More of the same tests.
There were about 12 of us there and it was nice not to be alone with this mystery disease. Many of these Cushies (mostly women) were getting bald, couldn’t walk, having strokes, had diabetes. One was blind, one had a heart attack while I was there. Towards the end of my testing period, I was looking forward to the surgery just to get this whole mess over with. While I was at NIH, I was gaining about a pound a day!
The MRI still showed nothing, so they did a Petrosal Sinus Sampling Test. That scared me more than the prospect of surgery. (This test carries the risk of stroke and uncontrollable bleeding from the incision points.) Catheters were fed from my groin area to my pituitary gland and dye was injected. I could watch the whole procedure on monitors. I could not move during this test or for several hours afterwards to prevent uncontrolable bleeding from a major artery. The test did show where the tumor probably was located. Also done were more sophisticated dexamethasone suppression tests where drugs were administered by IV and blood was drawn every hour (they put a heplock in my arm so they don’t have to keep sticking me). I got to go home for a weekend and then went back for the surgery – the Transsphenoidal Resection. I fully expected to die during surgery (and didn’t care if I did) so I signed my will and wrote last letters to those I wanted to say goodbye to. During the time I was home just before surgery, a college classmate of mine (I didn’t know her) did die at NIH of a Cushing’s-related problem. I’m so glad I didn’t find out until a couple months later!
November 3, 1987, the surgeon, Dr. Ed Oldfield, cut the gum above my front teeth under my upper lip so there is no scar. He used tiny tools and microscopes. My tumor was removed successfully. In some cases (not mine) the surgeon uses a plug of fat from the abdomen to help seal the cut. Afterwards, I was in intensive care overnight and went to a neurology ward for a few days until I could walk without being dizzy. I had some major headaches for a day or two but they gave me drugs (morphine) for those. Also, I had cotton plugs in my nostrils. It was a big day when they came out. I had diabetes insipidus (DI) for a little while, but that went away by itself – thank goodness!
I had to use a foam product called “Toothies” to brush my teeth without hitting the incision. Before they let me go home, I had to learn to give myself an injection in my thigh. They sent me home with a supply of injectible cortisone in case my level ever fell too low (it didn’t). I was weaned gradually off cortisone pills (scary). I now take no medications. I had to get a Medic Alert bracelet. I will always need to tell medical staff when I have any kind of procedure – the effects of my excess cortisone will remain forever.
I went back to the NIH for several follow-up visits of a week each where they did all the blood and urine testing again. After a few years NIH set me free. Now I go to my “outside” endocrinologist every year for the dexamethasone suppression test, 24-hour urine and regular blood testing.
As I get further away from my surgery, I have less and less chance that my tumor will grow back. I have never lost all the weight I gained and I still have the hair on my chin but most of my other symptoms are gone. I am still and always tired and need a nap most days. I do not, however, still need to take whole days off just to sleep.
I consider myself very lucky that I was treated before I got as bad as some of the others on my floor at NIH but think it is crazy that these symptoms are not taken seriously by doctors.
My story goes on and if you’re interested some is on this blog and some is here:
Forbes Magazine | MaryO’s bio | Cushing’s and Cancer Blog | Cushing’s Awareness Day Testimonial Archive |
Because of this experience in getting a Cushing’s diagnosis – and later, a prescription for growth hormone – I was concerned that there were probably other people not being diagnosed with Cushing’s. When I searched online for Cushing’s, all the sites that came up were for dogs and horses with Cushing’s. Not what I was looking for!
In July of 2000, I was talking with my dear friend Alice, who ran a wonderful menopause site, Power Surge, wondering why there weren’t many support groups online (OR off!) for Cushing’s. This thought percolated through my mind for a few hours and I realized that maybe this was my calling. Maybe I should be the one to start a network of support for other “Cushies” to help them empower themselves.
I wanted to educate others about the awful disease that took doctors years of my life to diagnose and treat – even after I gave them the information to diagnose me. I didn’t want anyone else to suffer for years like I did. I wanted doctors to pay more attention to Cushing’s disease.
The first website (http://www.cushings-help.com) went “live” July 21, 2000. It was just a single page of information. The message boards began September 30, 2000 with a simple message board which then led to a larger one, and a larger. Today, in 2010, we have over 7 thousand members. Some “rare disease”!
The message boards are stillactive and we have weekly online text chats, weekly live interviews, local meetings, conferences, email newsletters, a clothing exchange, a Cushing’s Awareness Day Forum, podcasts, phone support and much more. Because I wanted to spread the word to others not on “the boards” we have extended out to social networking sites – twitter groups, facebook groups, twines, friendfeeds, newsletters, websites, chat groups, multiply.com, and much, much more.
People are becoming more empowered and participating in their own diagnoses, testing and treatment. This have changed a lot since 1983!
When I had my Cushing’s over 40 years ago (AARRGGHH!), I never thought that I would meet another Cushing’s patient in real life or online. Back then, I’d never even been aware that there was anything like an “online”. I’m so glad that people struggling with Cushing’s today don’t have to suffer anymore thinking that they’re the only one who deals with this.
Because of my work on the websites – and, believe me it is a ton of work! – I have had the honor of meeting over a hundred other Cushies personally at local meetings, conferences, at NIH (the National Institutes of Health in Bethesda, MD where I had my final diagnosis and surgery). It occurred to me once that this is probably more than most endocrinologists will ever see in their entire career. I’ve also talked to countless others on the phone. Amazing for a “rare” disease!
I don’t know what pushed me in 1983, how I got the confidence and self-empowerment to challenge these doctors and their non-diagnoses over the years. I’m glad that I didn’t suffer any longer than I did and I’m glad that I have a role in helping others to find the medical help that they need.
What do *YOU* think? How are you becoming empowered?
Filed under: Clinical trials, Cushing's, General Health, Health Care, pituitary | Tagged: Alice Stamm (Dearest), buffalo hump, Cushing's, Cushing's Help, endocrinologist, gynecologist, hirsuitism, MaryO, message boards, moon face, nap, NIH, pituitary, Power Surge, Robin (staticnrg), surgery, tired, transsphenoidal, website, weight | 1 Comment »