Abstract
Objective
Activating mutation in Ubiquitin-specific peptidase (USP8) is identified to enhance cell proliferation and adrenocorticotropic hormone (ACTH) secretion from corticotroph pituitary adenoma. We investigated the USP8 variant status in a population of Iranian people with functional corticotroph pituitary adenoma (FCPA). Moreover, a systematic review was conducted to thoroughly explore the role of USP8 variants and the related pathways in corticotroph adenomas, genotype-phenotype correlation in USP8-mutated individuals with FCPA, and the potential role of USP8 and epidermal growth factor receptor (EGFR) as targeted therapies in PFCAs.
Methods
Genetic analysis of 20 tissue samples from 19 patients with PFCAs was performed using Sanger sequencing. Moreover, a systematic literature review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Scopus, web of Sciences, and Cochrane databases were searched. The last search was performed on 20 September 2023 for all databases.
Results
In our series, we found two somatic mutations including a 7-bp deletion variant: c.2151_2157delCTCCTCC, p. Ser718GlnfsTer3, and a missense variant: c.2159 C > G, p. Pro720Arg (rs672601311) in exon 14. The Systematic review indicated USP8 variant in 35% of corticotroph adenomas, with the highest frequency (25%) in 720 code regions, p. Pro720Arg. Data regarding the impact of USP8 mutational status on clinical characteristics and outcomes in FCPAs are inconsistent. Moreover, Pasireotide as well as inhibitors of EGFR such as Gefitinib and Lapatinib, as well as USP8 inhibitors including -ehtyloxyimino9H-indeno (1, 2-b) pyrazine-2, 3-dicarbonitrile, DUBs-IN-2, and RA-9 indicated promising results in treatment of corticotroph adenomas.
Conclusion
Although the USP8–EGFR system has been identified as the main trigger and target of corticotroph tumorigenesis, more precise multicenter studies are required to yield more consistent information regarding the phenotype-genotype correlation and to develop effective targeted therapies.
Introduction
Pituitary corticotroph adenoma accounts for 68% of endogenous hypercortisolism [1]. Prolonged exposure to high cortisol levels is associated with a variety of long-term complications, impaired quality of life, and increased mortality [2]. Transsphenoidal surgical excision is the treatment of choice. However, curative surgery is challenging with the initial remission rate of 65–85% and a high recurrence rate [3, 4].
The majority of functional corticotroph adenomas (FCAs) are sporadic. Although the genetic background is not well-established, potential candidate genes are proposed for tumor initiation and progression [5]. Hotspot mutations in ubiquitin-specific peptidase (USP8) are reported in 11–62% of sporadic corticotroph adenomas [6,7,8]. USP8 is a deubiquitinating enzyme that plays an important role in enhancing cell proliferation and regulating cell cycle [9]. The mutant USP8 was found to activate the epidermal growth factor receptor (EGFR) signaling pathway ultimately promoting adrenocorticotrophic hormone (ACTH) secretion [6]. Moreover, overexpression of EGFR and its signaling pathway components in pituitary corticotroph adenoma was reported [10]; and found to be positively associated with ACTH and cortisol levels as well as tumor recurrence [10]. These outcomes suggest that USP8 and EGFR are promising biomarkers for prediction of recurrence and can be used as targeted therapy.
Thus, we conducted a study to examine the USP8 and ubiquitin-specific peptidase 48 (USP48) variations in a group of Iranian people with Cushing’s disease (CD) and carried out a systematic review of the literature regarding the USP8/EGFR and their potential role in the clinical outcomes and targeted therapy in CD.
Methods
Case series
Study population
Paraffin-embedded blocks of pituitary tumor tissue from 19 patients with ACTH-secreting pituitary adenoma who underwent transsphenoidal surgery (TSS) between 2011 and 2019 were examined. The diagnosis of CD was based on clinical features and biochemical criteria [11]. The patients clinically suspected to CD were asked to collect urine free cortisol (UFC) in two separated times and underwent overnight dexamethasone suppression test (ODST). After confirmation of ACTH-dependent Cushing’s syndrome using measurement of ACTH level, a high-dose dexamethasone suppression test (HDDST) was performed to confirm the pituitary source of hypercortisolism. Patients with equivocal results or those with pituitary tumors less than 6 mm in size were undergone inferior petrosal sinus sampling (IPSS). Patient with clinical, biochemical, and radiological evidences of CD were undergone TSS. And eventually, corticotroph adenoma was confirmed using immunohistochemically staining of tumor tissue in all patients. The study was approved by the IUMS Research Ethics Committee (IR.IUMS.REC.1398.082). It was carried out under the declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice (ICH-GCP) guidelines, and informed consent was obtained from all patients.
DNA extraction and Sanger sequencing
A 10-µm thick section of formalin-fixed and paraffin-embedded (FFPE) tissue per sample was used for genomic DNA extraction. A molecular test was performed by amplification of USP8 and USP48 hotspot exons (exon 14 and exon 10, respectively) using conventional polymerase chain reaction (PCR). USP8 was amplified by two primer pairs; USP8_F1: AGCAGAATACTTTGGAGTGATTTC and USP8_R1: TTTGGAAGGTTCCCTATCCC with 251 bp product, USP8_F2: ACCCCTCCAACTCATAAAGC and USP8_R2: GAGTAGAAACTTTGAAATACAGCAC, with 220 bp product. A 240 bp fragment of USP48 was produced using; USP48_F: CCCGCTAAAGAATAAACAAACTC and USP48_R: GCATTCTAAAACATTTGCCTGC. PCR was done in 25 µl final volume (Ampliqon 2x PCR Mix) containing 0.5 µM of each primer and 30 ng of genomic DNA for 35 cycles (94 °C for 20 s, annealing 60 °C for 20 s and extension 72 °C for 20 s). The quality of PCR products was assessed by 2% agarose gel electrophoresis. Bidirectional Sanger sequencing was performed on an ABI DNA Analyzer (Applied Biosystems), The PCR primers were also used in the sequencing reaction. CodonCode Aligner software was used to analyze hotspot exome sequencing. Sequencing data quality was evaluated using Sanger electropherograms of both forward and reverse strands. The identified somatic mutations were analyzed in DNA taken from whole blood samples, but germline mutation was not detected.
Systematic review
Overview of the systematic literature review
We performed a systematic review of the literature to identify all published papers that reported the frequency of the USP8 variant and the related pathways in corticotroph pituitary adenomas, detailed clinical presentation and outcomes of patients with and without USP8 mutation and examined the USP8 and EGFR as targeted therapy.
Search strategy
We searched the PubMed, Scopus, web of Sciences, and Cochrane databases. The date of the last search was 20 September 2023 for all databases. We did not apply any language restrictions. Search terms included: “Cushing disease”, “Cushing’s disease”, “Corticotroph adenoma”, “Cushing adenoma”, “Client Cushing disease”, “Atypical corticotroph tumor”, “Corticotroph carcinoma”, “Normal pituitary”, “Corticotroph adenoma”, “Corticotroph Tumor”, “Pituitary ACTH Hypersecretion”, “ACTH-Secreting Pituitary Adenoma”, “Mutation”, “Germline mutation”, “Sporadic mutation”, USP8, “ubiquitin specific peptidase 8”, USP48, “ubiquitin specific peptidase 48”, “Epidermal growth factor”, EGF, “Epidermal growth factor receptor” EGFR, Biomarker.
Inclusion and exclusion criteria
All published papers including original articles, case reports, and case series were included in this systematic review provided that they have reported the frequency of USP8 variant or EGFR expression in corticotroph pituitary adenomas, compared the clinical presentation and outcomes of patients with and without USP8 variant, or examined USP8 or EGFR as treatment targets in CD. Studies applying any type of tissue namely resected human pituitary adenoma tissue, primary cell cultures, cell lines, and transfected cells were included. Articles were excluded if they included different types of pituitary tumors and did not separately analyze corticotroph adenomas, or if they were written in any language other than English.
Results
Case series
Baseline characteristics of the participants
This study included 19 patients of whom 63% (n = 12) were women. They aged between 17 and 65 years. Baseline cortisol ranged between 20 and 43 mic/dl. The ACTH level ranged between 34 and 164 pg/ml. The basal UFC ranged between 316 and 1153 mic/24 h. All patients presented with micro-adenoma except for two patients, one man and one woman (supplementary Table 1).
Frequency of USP8 gene variants
Sanger sequencing of 20 CD tumors revealed two heterozygous pathogenic variants in 2 samples: the 7-bp deletion variant, c.2151_2157delCTCCTCC, p. Ser718GlnfsTer3 was found in one patient; another patient showed the missense variant, c.2159 C > G, p. Pro720Arg (rs672601311) in exon 14. The pathogenic variants were found only in tumor tissue. Targeted sequencing (exon 10) of USP48 did not detect any pathogenic variant. The somatic variations in our study are in the catalytic conserved domain of USP8 protein and lead to disruption of the interaction between USP8 catalytic domain and 14-3-3 protein (Fig. 1).
Sanger sequencing of pathogenic variants in USP8 hotspot exon. (A, B) bi-directional sequencing of heterozygous missense variant, c.2159 C > G, in tissue sample, (C) A Sanger sequencing chromatogram of the blood sample detected no germline c.2159 C > G mutation. (D) Sanger sequencing chromatograms confirm the presence of heterozygous deletion (c.2151_2157delCTCCTCC) in tissue sample of patient II
Clinical outcomes after surgery
All patients achieved biochemical and structural cures after surgery except for one man and one woman who suffered from persistent disease because the tumors were not completely resected due to invasion into the cavernous sinus. They underwent radiotherapy after surgery. These two patients did not show the USP8 variant. Moreover, one man without evidence of the USP8 variant and the two women with the USP8 variant presented with recurrence after initial remission. They presented with micro-adenoma before surgery (supplementary Table 1).
Systematic review
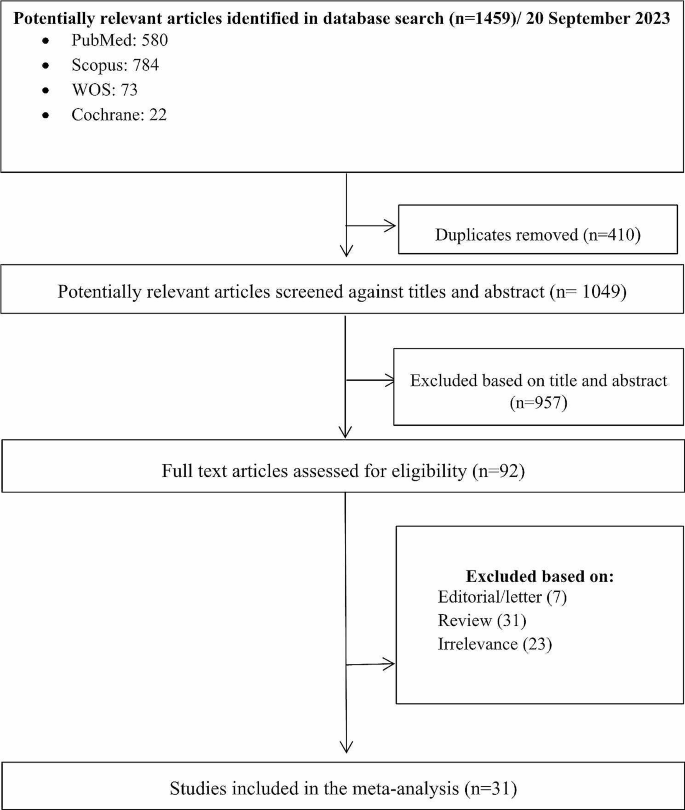
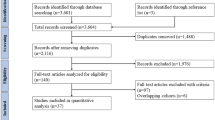
The search yielded 1459 initial results. Upon removing the duplications (n = 410), 1049 studies were reviewed based on the relevancy of their titles and abstracts. Having excluded 957 articles, 92 studies were selected for full-text review. After an in-depth review, 31 articles were selected based on the inclusion and exclusion criteria. A PRISMA diagram detailing the search results is shown in Fig. 2.
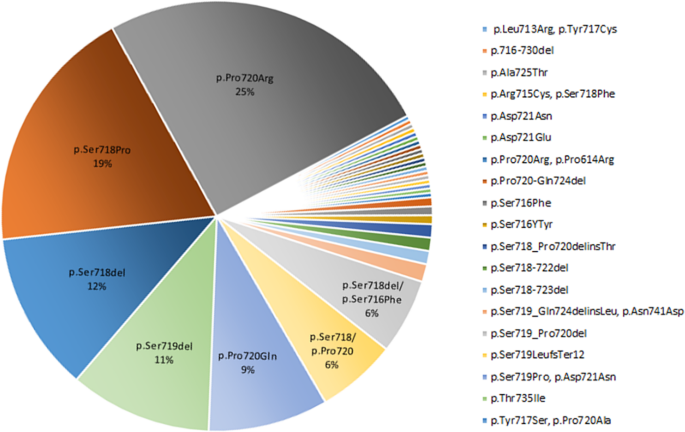
In this systematic review we extracted the information regarding the USP8 variant and the EGFR system in corticotroph adenomas. The USP8 variant was found in 460 individuals with FCPA accounting for 35% of the population included in the related published series (Table 1). Moreover, the highest frequency of missense mutation was found in the 720 code region, p.Pro720Arg (25%), followed by 19% in p.Ser718Pro (Fig. 3). In addition, the frequency of frame-shift and in-frame deletion observed in p.Ser718del and p.Ser719del was 12% and 11%, respectively (Fig. 3).
Summary of USP8 mutations in patients with CD in selected studies
USP8 variants and the related pathways in corticotroph adenomas
In a study of 42 patients with corticotroph adenomas, USP8 variants were as follows: p. P720R (found in five patients), p. S718P (found in two patients), p. P720Q (found in two patients), p. S716Y (found in one patient), and p. S716F (found in one patient) [12]. Another genetic study demonstrated mutated USP8 deubiquitinating EGFR more effectively than wild type USP8. Some variants namely p.S718del, p.718SP, and p.P720R have higher deubiquitinated activity, while others including p.S718C, p.L713R, and p.Y717C showed similar activity compared to the wild type. These variants have been shown to increase the catalytic and proteolytic activity of USP8, which ultimately leads to the activation of the EGFR pathway. High EGFR levels, in turn, stimulate POMC gene transcription and increase plasma ACTH levels [6]. In the study of Seata, the USP8 variant was found in 23% of corticotroph adenomas. The variants were heterozygous, including p.S718, p.P720 (n = 18), p.S719del (n = 10), and p.P720_723 del (n = 1). Moreover, a comparison of 5 USP8 mutant vs. 34 wild-type specimens indicated different gene expression profile. According to the results, 2 genes involving in EGF signaling, CMTM8 (CKLFlike MARVEL transmembrane domain containing 8) and MAPK15 (mitogen-activated protein kinase 15), were upregulated in USP8 variant carriers [13]. Bujko et al. found USP8 mutation in 31.3% of patients with FCA and silent corticotroph adenomas (SCA). In-frame and missense mutations were p.Ser718del (7 patients), p.Pro720Arg (5 patients), p.Ser718Pro (2 patients) and p.Pro720Gln (one patient). USP8-mutated adenomas showed higher level of POMC, CDC25A, MAPK4 but lower level of CCND2, CDK6, CDKN1B than USP8-wild-type tumors [14].
Another study investigated the molecular pathogenesis of the spectrum of corticotroph adenomas, including CD, SCA, CCA (Crooke cell adenomas), and ACTH-producing carcinoma using whole exome sequencing. The patients with ACTH-producing carcinoma showed the highest number of variants in USP8, EGFR, TP53, AURKA, CDKN1A, and HSD3B1 genes. The USP8 variant was found in c.2159 C > G (p.Pro720Arg) and was positively correlated to the tumor size. However, the USP8 variant was not present in any of the patients with CD [15].
Martins and colleagues conducted a study to investigate the USP8 variant and its contribution to gene expression of cell cycle regulators including P27/CDKN1B, CCNE1, CCND1, CDK2, CDK4, and CDK6 in 32 corticotroph adenoma. They identified variants in certain hotspot exons, namely p.720R (found in five patients), p.S718del (found in three patients), p.S718P (found in one patient), and p.S719_T723del (found in one patient). Moreover, there was no difference regarding the gene expression of the cell cycle regulators CDKN1B (P27), CCNE1 (CYCLIN-E1), CCND1 (CYCLIN-D1), CDK2, CDK4, and CDK6 according to USP8 variant status [16]. Another study investigating the USP8 variants and genes involved in cell cycle regulation observed USP variants including p. P720R (n = 8), p.720Q (n = 2), p. S718SP (n = 2), and an in-frame deletion at the 719 position (n = 8). However, USP8-mutated tumors showed lower CDKN1B, CDK6, CCND2 and higher CDC25A expression. They also observed a significantly lower level of p27 in USP8-mutated tumors as compared to the wild-type ones [17].
A comprehensive study determined the presence of EGFR at the protein and mRNA levels in different pituitary adenomas. The highest incidence of EGFR expression was found in corticotroph adenomas. The corticotroph adenomas with EGFR expression did not show p27 immunoreactivity [18].
DNA methylation regulates promoter activities. The study by Araki et al. identified a novel regulatory region in the human POMC gene which functions as a second promoter. Moreover, they indicated that this region is highly methylated in SCAs and highly demethylated in FCAs and ectopic ACTH-secreting tumors. They also demonstrated demethylation of the second promoter is associated with aggressive features of FCAs independent of the USP8 variant or EGFR signaling. In contrast, the first promoter was highly demethylated in USP8-mutated FCAs [19]. Weigand et al. indicated that p27/kip1 protein expression significantly decreased in USP8-mutated adenomas compared to the wild-type USP8 tumors. Moreover, higher expression of heat shock protein 90 (HSP90) and an increase in the phosphorylation of the transcription factor CREB was observed in mutated-USP8 adenomas [20]. Achaete-scute complex homolog 1 (ASCL1) plays an important role in cell proliferation and also regulates POMC in the cell line. In a recent study, genetic analysis of corticotroph adenomas using RNA-seq and IHC showed an increase in ASCL1expression and protein levels in both mutated and wild type USP8 among CD patients [21].
Genotype-phenotype correlation in USP8-mutated individuals with functional corticotroph adenoma
Sanger sequencing of 120 FCPAs indicated the somatic USP8 variant more frequently in women than in men, which was associated with a significant lower size and higher ACTH level. Moreover, compared to the wild-type tumors, the USP8-mutated ones display a higher level of EGFR expression with a higher staining intensity. The initial remission rate and the recurrence rate in patients initially receiving remission were comparable in both groups [7]. Another study of patients with 134 functional and 11 silent corticotroph adenomas demonstrated somatic USP8 variants only in functional adenomas, none of them occurred in silent adenomas. The USP8 variant in adults was associated with lower age, and predominantly occurred in women. Moreover, the presence of USP8 variant was inversely associated with remission [22]. In a cohort of 42 pediatric patients with FCA, five different USP8 variants (three missenses, one frame-shift, and one in-frame deletion) were identified. None of the patients were found to have gremlin USP8 variants. Patients with somatic USP8 variant were significantly older than those with wild-type USP8. However, there was no significant difference in terms of preoperative hormonal profile and tumor invasiveness between the two groups. However, somatic USP8 mutated patients showed a higher rate of recurrence after a mean follow-up of 34.7 months [23].
In a cohort of 48 FCA, patients with the USP8 variant had significantly higher levels of preoperative urine-free cortisol (UFC). But there was no difference in preoperative ACTH and cortisol level between USP8-mutated and wild-type groups. Although initial remission rate was similar in both groups, patients with USP8 variant revealed a significantly higher rate of recurrence within 10 years follow-up, with a significantly shorter time to recurrence [24]. USP8-mutated FCA patients presented with a significantly larger size of adenoma in a retrospective study. But preoperative hormonal profile and the remission rate were similar in both groups [16]. Retrospective genetic analysis of 92 FCA patients indicated that the USP8 variant was significantly higher in women than men. There was no significant difference in preoperative hormonal profile and tumor size between USP8-mutated and wild-type groups. USP8-mutated carriers were more likely to achieve surgical remission. However, after 10 years follow-up, the recurrence rate was similar in the both groups [25]. A Retrospective study of patients with 30 functional and 20 silent corticotroph adenomas showed USP8 variants in 11 and 2 adenomas, respectively. There was no difference in sex, age, preoperative hormonal profile, and size of the adenomas between patients with and without USP8 variants. However, the USP8-mutated tumors revealed a higher rate of invasiveness. Furthermore, somatostatin receptor 5 (SSRT5) was more frequent in USP8-mutated adenomas [26]. In a retrospective study of FCA patients found no difference considering age at the presentation and hormonal profile between patients with and without USP8 variants. However, macro-adenoma was more frequently seen in USP8-mutated patients. Although initial remission rate was similar in the both groups, after a median 5 (2–8) years of follow-up, USP8-mutated carriers were more likely to develop recurrence [27]. The study conducted by Bujko et al., comparing patients with USP8 mutated and wild-type corticotroph adenomas, demonstrated no difference in age, sex, preoperative hormonal profile, tumor invasiveness, proliferation index, and histology (sparsely vs. densely granulation) between the two groups. However, the USP8-mutated patients showed a higher rate of remission [28].
A cohort of Asian-Indian patients with CD identified that there was no significant difference considering age, sex, tumor size, tumor invasion, and preoperative hormonal profile of the participants with and without the USP8 variant. Moreover, the initial remission rate and long-term recurrence, after a mean follow-up of 25.3 ± 13.6 months, were also comparable in both groups [29]. Liu et al. investigated the expression of EGFR and its signaling pathways in FCAs. They demonstrated that EGFR was overexpressed in 29 of 52 patients with FCA. Moreover, the EGFR signal transducing molecules p-EGFR, p-Akt and p-Erk were upregulated in EGFR-overexpressing adenomas but not in EGFR-negative adenomas. Moreover, the expression of EGFR was positively correlated with ACTH and cortisol levels but not with age, sex, or adenoma size. After a mean follow-up of 42.8 months, 22 patients had tumor recurrence. The EGFR expression was positively associated with the recurrence rate [10].
USP8 and EGFR as potential therapeutic targets in functional corticotroph adenoma
Our systematic search yields nine studies investigating the possible role of the USP8 variant in response to the medications. Four studies evaluated the presence of SSTR5 receptors in USP8– mutated tumors. Genetic analysis of FCAs from a cohort of 39 functional and 23 silent corticotroph adenoma indicated that there was no difference regarding the age of the participants, as well as hormonal profile, size, and invasiveness of the tumor between patients with and without USP8 variants. However, USP8-mutated adenomas showed significantly higher SSRT5 expression compared to the wild-type ones [26].
In a cohort study, USP8-mutated FCA patients were dominantly women and showed lower ACTH levels and smaller tumor size, but no difference in cortisol level. Remission rate was significantly higher in USP8-mutated patients compared to the wild-type ones. Moreover, USP8-mutated adenomas were more likely to express SSTR5 [30]. Genetic analysis of 51 FFPE tumors (21 USP8-mutated and 30 wild-type) indicated significantly higher SSTR5 immunoreactivity score in USP8-mutated tumors, regardless of mutation type. Moreover, in vitro study of 24 corticotroph tumors freshly obtained after TSS indicated a significantly better response to Pasireotide treatment, defined as suppression of ACTH secretion, in human corticotroph tumors carrying USP8 variants [31].
A more recent study aimed to investigate the impact of USP8 variants on in vitro response to Pasirotide in primary cultures obtained from 7 FCAs and also in murine corticotroph tumor cells. USP8 variant in both primary cultured cells and AtT20 cells was associated with higher SSTR5 expression. Moreover, this study indicated although associated with SSTR5 upregulation, mutations at the amino acid 718 of USP8 are not associated with a favorable response to pasireotide, whereas USP8 variants at the amino acid 720 might preserve pasireotide responsiveness [32].
Inhibition of EGFR using Gefitinib, a tyrosine kinase inhibitor, in surgically resected human and canine corticotroph cultured tumors suppressed expression of POMC. Moreover, Blocking EGFR activity in mice attenuated POMC expression, inhibited corticotroph tumor cell proliferation, and induced apoptosis [33]. Araki et al. conducted a study to investigate the utility of EGFR as a therapeutic target for CD. EGFR expression was observed by 2.5 months in transgenic (Tg) mice; and aggressive ACTH-secreting pituitary adenomas with features of Crooke’s cells developed by 8 months with 65% penetrance observed. Moreover, they used the EGFR tyrosine kinase inhibitor Gefitinib to confirm reversibility of EGFR effects on ACTH. Gefitinib suppressed tumor POMC expression and downstream EGFR tumor signaling. Plasma ACTH level and pituitary tumor size was significantly lower in Gefitinib group [34].
Another experimental study investigated the effect of Lapatinib, a potent tyrosine kinase inhibitor, on ACTH production and cell proliferation in AtT-20 mouse corticotroph tumor cells. Lapatinib inhibits EGFR. In this study, Lapatinib decreased proopiomelanocortin (POMC) mRNA levels and ACTH levels in AtT-20 cells and also inhibited cell proliferation and induced apoptosis. Inhibition of EGFR signaling contributes to the inhibition of ACTH production and cell proliferation in corticotroph adenomas [35].
The effect of a potent and selective Jak2 inhibitor, SD1029, on ACTH production and proliferation investigated in mouse AtT20 corticotroph tumor cells. They observed that Jak2 inhibitor SD1029 decreased both POMC transcript levels and basal ACTH levels. These in vitro experiments suggest the Jak2 inhibitor suppresses both the autonomic synthesis and release of ACTH in corticotroph tumor cells. SD1029 was also found to inhibit AtT20-cell proliferation. In addition, SD1029 decreased and increased PTTG1 and GADD45β transcript levels, respectively. They seem to contribute, in part, in the Jak2-induced suppression of cell proliferation and ACTH synthesis [36]. An experimental study examined the effect of USP8 inhibitor on EGFR expression level, and cell viability using AtT20 cells treated with 9-ehtyloxyimino9H-indeno (1, 2-b) pyrazine-2,3-dicarbonitrile, a synthesized USP8 inhibitor. This study demonstrated that treatment with USP8 inhibitor, 9‑ehtyloxyimino9H‑indeno(1,2‑b) pyrazine‑2,3 dicarbonitrile, suppresses ACTH secretion, cell viability, and promotes cell apoptosis in AtT20 cells suggesting that USP8 inhibitor could be a new therapeutic candidate for CD [37].
Kageyama et al. investigated the effects of a potent USP8 inhibitor, DUBs-IN-2, on ACTH production and cell proliferation in mouse corticotroph tumor (AtT-20) cells. DUBs-IN-2 decreased Proopiomelanocortin (POMC) mRNA and ACTH levels. Furthermore, DUBs-IN-2 decreased At-20 cell proliferation and induced apoptosis in corticotroph tumor cells [38]. Another study explored the potential effect of the USP8 inhibitor RA-9 on USP8-WT human tumor corticotroph cells and murine AtT-20 cells. RA-9 significantly decreased cell proliferation and increased cell apoptosis in AtT-20 cells. Moreover, RA-9 reduced ACTH release by USP8-mutant cells. The combined treatment with RA-9 and pasireotide resulted in more efficient in inhibiting ACTH secretion compared with RA-9 or pasireotide alone. Furthermore, similar to pasireotide, RA-9 was able to significantly reduce phospho- ERK1/2 levels in both AtT-20 cells and primary cultured cells from corticotropinomas [39].
Another study, investigating the USP8 variants and genes involved in cell cycle regulation, looked for the role of USP8 variants or a changed p27 level in the response to Palbociclib, Flavopiridol, and Roscovitine, in vitro, using murine corticotroph AtT-20/D16v-F2 cells. They did not found any significant difference in cell viability or cell proliferation between the AtT-20/D16v-F2 cells overexpressing wild-type and mutated USP8 that were treated with cell cycle inhibitors. There was also no difference in the response to inhibitors of CKDs in the cells with overexpression of p27 and control cells [17].
Analytical conclusion
In our series, we found two USP8 variants including a 7-bp deletion variant, c.2151_2157delCTCCTCC, p. Ser718GlnfsTer3, and a missense variant, c.2159 C > G, p. Pro720Arg (rs672601311) in exon 14. Moreover, the systematic review of the published data indicated that 35% of corticotroph adenomas harbor USP8 variant the most of which was found in the 720 code region, p. Pro720Arg. Similar to the most previous studies, the USP-8 mutated patients were women, presented with micro-adenoma and experienced recurrence after initial remission.
We systematically reviewed the literature regarding the USP8 variant in corticotroph adenomas and classified the results into three categories; including USP8 variants and the related pathways, genotype-phenotype correlation in USP8-mutated individuals, and USP8 and EGFR as potential therapeutic targets.
Different USP8 variants are identified in corticotroph adenomas. Activation of the EGFR pathway is a well-established consequence of USP8 variants [6, 15]. But there is inconsistency regarding the role of USP8 variants in cell cycle regulation in corticotroph adenomas. Some studies showed no difference in the gene expression of the cell cycle regulators CDKN1B (P27), CCNE1 (CYCLIN-E1), CCND1 (CYCLIN-D1), CDK2, CDK4, and CDK6 according to USP8 variant status [21]; while the others indicated USP8-mutated tumors have lower CDKN1B, CDK6, CCND2 and higher CDC25A expression [20]. Moreover, demethylation of the first promoter is affected with USP8 variant status [19]. However, more studies are required to establish the pathway underlying the USP8 variants.
Data regarding sex, age, hormonal level, tumor size, and clinical outcomes in USP8-mutated individuals with FCA are relatively consistent among different studies. The USP8 variant seems to be associated with younger age and is more likely to occur in women. Meta-analysis of data from ten series indicated USP8 variant is 2.63 times higher in women than in men [40]. Since CD is more prevalent in young women, the potential effect of estrogen on the growth of USP8-mutant corticotroph cells has been hypothesized. There is evidence that corticotroph cells express estrogen receptors [41]. Moreover, in vitro studies indicated estrogen can stimulate corticotroph cell proliferation mediated by EGFR signaling pathways [42]. More precise studies are required to better explain the age-sex distribution of USP8 variant in patients with CD.
Results regarding the hormonal pattern among the series are partly controversial. Two series indicated significantly higher levels of ACTH and UFC in USP8-mutated patients compared to the wild-type ones [7, 24]. Moreover, one study demonstrated the expression levels of EGFR were positively correlated with ACTH and cortisol levels [10]. Conversely, one study showed a significantly lower ACTH level in patients with the USP8 variant [30]. However, in a systematic analysis of the two series the correlation of UFC and USP8 variant did not reach a significant difference, this might be due to the small number of cases included in the analysis [40].
There are also some discrepancies on tumor size and invasiveness in USP8-mutated tumors. Some studies indicated a significant smaller size in USP8-mutated tumors, while others showed a significant larger size in USP8-mutated tumors. But some study found no significant difference regarding tumor size and invasiveness between USP8-mutated and wild-type tumors. A recent systematic analysis of magnetic resonance imaging (MRI) findings from individuals with CD indicated USP8-mutated tumors are more likely to be less than 10 mm compared to wild-type ones [40]. Moreover, a cohort of 60 patients with FCA indicated smaller tumor size and less invasiveness in USP8-mutated tumors [30]. In contrast to these findings, a cohort of Brazilian patients observed a tendency toward more somatic USP8 variant in tumors more than 10 mm in size [40]. These discrepancies might be due to the different methods used for extraction of MRI data.
Considering the clinical outcomes, most studies indicated a higher remission rate except for one that showed a significantly lower rate of remission in USP8-mutated patients [22, 25, 28, 30]. Moreover, some studies demonstrated a higher rate of recurrence in carriers of USP8 variant [24, 27, 42]. However, other studies found no significant difference neither in the initial remission nor in the late recurrence rate between the carriers of USP8 variant and the individuals with wild-type USP8. The inconsistency in the results might be due to the lack of a systematic protocol for evaluation of these patients. Moreover, the number of patients included in the different studies was relatively low. Further multicenter prospective studies with the same protocol are required to yield more consistent information regarding the influence of USP8 variant on the clinical presentation as well as early and late outcomes of FCAs.
There are promising studies regarding USP8-targeted therapy. We found evidence that USP8-mutated tumors have higher SSRT5 expression [30, 31]. Moreover, in vitro studies demonstrated that Pasirotide suppressed ACTH secretion significantly more in the USP8-mutated tumors than in wild-type ones [31]. These evidences suggest that USP8 mutational status could be used as a marker of Pasirotide response in CD. Furthermore, USP8-mutated tumors are more likely to express EGFRs compared to the wild-type ones [6]. Inhibition of EGFR using Gefitinib and Lapatinib has been associated with promising results regarding the EGFR-targeting therapy in CD [33,34,35]. Moreover, experimental studies of two USP8 inhibitors, 9‑ehtyloxyimino9H‑indeno (1,2‑b) pyrazine‑2,3 dicarbonitrile and DUBs-IN-2, have shown their potential to suppress POMC expression and ACTH secretion, decrease cell proliferation, and promote apoptosis [37, 38].
In summary, the studies investigated the association of USP8– variants and clinical manifestations as well as clinical outcomes of the corticotroph adenomas are partly inconsistent. More precise multicenter studies are required to yield more consistent information regarding the phenotype-genotype correlation and to develop effective targeted therapies.
Data availability
The datasets used and/or analyzed during the current atudy are available from the corresponding author on reasonable request.
Abbreviations
- ABI:
- Applied Biosystems
- ACTH:
- Adrenocorticotropic Hormone
- CCA:
- Crooke Cell Adenomas
- CD:
- Cushing’s Disease
- DNA:
- Deoxyribonucleic Acid
- EGFR:
- Epidermal Growth Factor Receptor
- Erk:
- Extracellular Signal-Regulated Kinases
- FCAs:
- Functional Corticotroph Adenomas
- FCPA:
- Functional Corticotroph Pituitary Adenoma
- FFPE:
- Formalin-Fixed And Paraffin-Embedded
- ICH-GCP:
- International Conference On Harmonization Of Good Clinical Practice
- IHC:
- Immunohistochemistry
- MRI:
- Magnetic Resonance Imaging
- PCR:
- Polymerase Chain Reaction
- PRISMA:
- Preferred Reporting Items For Systematic Reviews And Meta-Analyses
- RNA-seq:
- RNA Sequencing
- SCA:
- Silent Corticotroph Adenomas
- TSS:
- Transsphenoidal Surgery
- USP8:
- Ubiquitin-Specific Peptidase
- USP48:
- Ubiquitin Specific Peptidase 48
References
-
Uwaifo GI, Hura DE. Hypercortisolism. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK551526/.
-
Pivonello R, Simeoli C, Di Paola N, Colao A. Cushing’s disease: adrenal steroidogenesis inhibitors. Pituitary. 2022;25(5):726–32.
-
Balomenaki M, Vassiliadi DA, Tsagarakis S. Cushing’s disease: risk of recurrence following trans-sphenoidal surgery, timing and methods for evaluation. Pituitary. 2022;25(5):718–21.
-
Mallari RJ, Thakur JD, Barkhoudarian G, Eisenberg A, Rodriguez A, Rettinger S, Cohan P, Nieman L, Kelly DF. Diagnostic pitfalls in Cushing Disease: Surgical Remission Rates, Test Thresholds, and lessons learned in 105 patients. J Clin Endocrinol Metab. 2022;107(1):205–18.
-
Seltzer J, Ashton CE, Scotton TC, Pangal D, Carmichael JD, Zada G. Gene and protein expression in pituitary corticotroph adenomas: a systematic review of the literature. Neurosurg Focus. 2015;38(2):E17.
-
Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31–8.
-
Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, Mao Y, Li YM, Hu RG, Zhang ZY, et al. Gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306–17.
-
Rebollar-Vega RG, Zuarth-Vázquez JM, Hernández-Ramírez LC. Clinical spectrum of USP8 pathogenic variants in Cushing’s Disease. Arch Med Res. 2023;54(8):102899.
-
Naviglio S, Mattecucci C, Matoskova B, Nagase T, Nomura N, Di Fiore PP, Draetta GF. UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J. 1998;17(12):3241–50.
-
Liu X, Feng M, Dai C, Bao X, Deng K, Yao Y, Wang R. Expression of EGFR in Pituitary Corticotroph Adenomas and its RelationshipWith Tumor Behavior. Front Endocrinol (Lausanne). 2019;10:785.
-
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40.
-
Ballmann C, Thiel A, Korah HE, Reis AC, Saeger W, Stepanow S, Köhrer K, Reifenberger G, Knobbe-Thomsen CB, Knappe UJ, Scholl UI. USP8 Mutations in Pituitary Cushing Adenomas-Targeted Analysis by Next-Generation Sequencing. J Endocr Soc. 2018;2(3):266–278.
-
Sesta A, Cassarino MF, Terreni M, Ambrogio AG, Libera L, Bardelli D, et al. Ubiquitin-specific protease 8 mutant corticotrope Adenomas Present Unique secretory and molecular features and shed light on the role of Ubiquitylation on ACTH Processing. Neuroendocrinology. 2020;110(1–2):119–29.
-
Bujko M, Kober P, Boresowicz J, Rusetska N, Paziewska A, Dąbrowska M, Piaścik A, Pękul M, Zieliński G, Kunicki J, et al. USP8 mutations in corticotroph adenomas determine a distinct gene expression profile irrespective of functional tumour status. Eur J Endocrinol. 2019;181(6):615–27.
-
Andonegui-Elguera S, Silva-Román G, Peña-Martínez E, Taniguchi-Ponciano K, Vela-Patiño S, Remba-Shapiro I, Gómez-Apo E, Espinosa-de-Los-Monteros AL, Portocarrero-Ortiz LA, Guinto G, et al. The genomic Landscape of Corticotroph tumors: from Silent Adenomas to ACTH-Secreting Carcinomas. Int J Mol Sci. 2022;23(9):4861.
-
Martins CS, Camargo RC, Coeli-Lacchini FB, Saggioro FP, Moreira AC, de Castro M. USP8 mutations and cell cycle regulation in Corticotroph Adenomas. Horm Metab Res. 2020;52(2):117–23.
-
Mossakowska BJ, Rusetska N, Konopinski R, Kober P, Maksymowicz M, Pekul M, Zieliński G, Styk A, Kunicki J, Bujko M. The expression of cell cycle-related genes in USP8-Mutated corticotroph neuroendocrine pituitary tumors and their possible role in cell cycle-targeting treatment. Cancers (Basel). 2022;14(22):5594.
-
Theodoropoulou M, Arzberger T, Gruebler Y, Jaffrain-Rea ML, Schlegel J, Schaaf L, Petrangeli E, Losa M, Stalla GK, Pagotto U. Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J Endocrinol. 2004;183(2):385–94.
-
Araki T, Tone Y, Yamamoto M, Kameda H, Ben-Shlomo A, Yamada S, Takeshita A, Yamamoto M, Kawakami Y, Tone M, Melmed S. Two distinctive POMC promoters modify Gene expression in Cushing Disease. J Clin Endocrinol Metab. 2021;106(9):e3346–63.
-
Weigand I, Knobloch L, Flitsch J, Saeger W, Monoranu CM, Höfner K, Herterich S, Rotermund R, Ronchi CL, Buchfelder M, et al. Impact of USP8 gene mutations on protein deregulation in Cushing Disease. J Clin Endocrinol Metab. 2019;104(7):2535–46.
-
Chen Z, Jia Q, Zhao Z, Zhang Q, Chen Y, Qiao N, Ye Z, Ji C, Zhang Y, He W, et al. Transcription factor ASCL1 acts as a novel potential therapeutic target for the treatment of the Cushing’s Disease. J Clin Endocrinol Metab. 2022;107(8):2296–306.
-
Perez-Rivas LG, Theodoropoulou M, Ferraù F, Nusser C, Kawaguchi K, Stratakis CA, Faucz FR, Wildemberg LE, Assié G, Beschorner R, et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in Adenomas causing Cushing’s Disease. J Clin Endocrinol Metab. 2015;100(7):E997–1004.
-
Faucz FR, Tirosh A, Tatsi C, Berthon A, Hernández-Ramírez LC, Settas N, Angelousi A, Correa R, Papadakis GZ, Chittiboina P, et al. Somatic USP8 gene mutations are a Common cause of Pediatric Cushing Disease. J Clin Endocrinol Metab. 2017;102(8):2836–43.
-
Albani A, Pérez-Rivas LG, Dimopoulou C, Zopp S, Colón-Bolea P, Roeber S, Honegger J, Flitsch J, Rachinger W, Buchfelder M. The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin Endocrinol (Oxf). 2018.
-
Losa M, Mortini P, Pagnano A, Detomas M, Cassarino MF, Pecori Giraldi F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine. 2019;63(2):240–6.
-
Castellnou S, Vasiljevic A, Lapras V, Raverot V, Alix E, Borson-Chazot F, Jouanneau E, Raverot G, Lasolle H. SST5 expression and USP8 mutation in functioning and silent corticotroph pituitary tumors. Endocr Connect. 2020;9(3):243–53.
-
Treppiedi D, Barbieri AM, Di Muro G, Marra G, Mangili F, Catalano R, Esposito E, Ferrante E, Serban AL, Locatelli M, et al. Genetic profiling of a cohort of Italian patients with ACTH-Secreting pituitary tumors and characterization of a novel USP8 gene variant. Cancers (Basel). 2021;13(16):4022.
-
Bujko M, Kober P, Boresowicz J, Rusetska N, Zeber-Lubecka N, Paziewska A, Pekul M, Zielinski G, Styk A, Kunicki J, Ostrowski J, et al. Differential microRNA expression in USP8-Mutated and wild-type Corticotroph Pituitary tumors reflect the difference in protein ubiquitination processes. J Clin Med. 2021;10(3):375.
-
Abraham AP, Pai R, Beno DL, Chacko G, Asha HS, Rajaratnam S, Kapoor N, Thomas N, Chacko AG. USP8, USP48, and BRAF mutations differ in their genotype-phenotype correlation in Asian Indian patients with Cushing’s disease. Endocrine. 2022;75(2):549–59.
-
Hayashi K, Inoshita N, Kawaguchi K, Ibrahim Ardisasmita A, Suzuki H, Fukuhara N, Okada M, Nishioka H, Takeuchi Y, Komada M, et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur J Endocrinol. 2016;174(2):213–26.
-
Albani A, Perez-Rivas LG, Tang S, Simon J, Lucia KE, Colón-Bolea P, Schopohl J, Roeber S, Buchfelder M, Rotermund R, et al. Improved pasireotide response in USP8 mutant corticotroph tumours in vitro. Endocr Relat Cancer. 2022;29(8):503–11.
-
Treppiedi D, Marra G, Di Muro G, Esposito E, Barbieri AM, Catalano R, Mangili F, Bravi F, Locatelli M, Lania AG, et al. P720R USP8 mutation is Associated with a better responsiveness to Pasireotide in ACTH-Secreting PitNETs. Cancers. 2022;14(10):2455.
-
Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712–21.
-
Araki T, Liu X, Kameda H, Tone Y, Fukuoka H, Tone M, Melmed S. EGFR Induces E2F1-Mediated Corticotroph Tumorigenesis, J Endocr Soc. 2017; 2017;1(2):127–143.
-
Asari Y, Kageyama K, Sugiyama A, Kogawa H, Niioka K, Daimon M. Lapatinib decreases the ACTH production and proliferation of corticotroph tumor cells. Endocr J. 2019;66(6):515–22.
-
Asari Y, Kageyama K, Nakada Y, Tasso M, Takayasu S, Niioka K, Ishigame N, Daimon M. Inhibitory effects of a selective Jak2 inhibitor on adrenocorticotropic hormone production and proliferation of corticotroph tumor AtT20 cells. Onco Targets Ther. 2017;10:4329–38.
-
Jian FF, Li YF, Chen YF, Jiang H, Chen X, Zheng LL, Zhao Y, Wang WQ, Ning G, Bian LG, Sun QF. Inhibition of Ubiquitin-specific peptidase 8 suppresses adrenocorticotropic hormone production and tumorous corticotroph cell growth in AtT20 cells. Chin Med J. 2016;129(17):2102–8.
-
Kageyama K, Asari Y, Sugimoto Y, Niioka K, Daimon M. Ubiquitin-specific protease 8 inhibitor suppresses adrenocorticotropic hormone production and corticotroph tumor cell proliferation. Endocr J. 2020;67(2):177–84.
-
Treppiedi D, Di Muro G, Marra G, Barbieri AM, Mangili F, Catalano R, Serban A, Ferrante E, Locatelli M, Lania AG, et al. USP8 inhibitor RA-9 reduces ACTH release and cell growth in tumor corticotrophs. Endocr Relat Cancer. 2021;28(8):573–82.
-
Wanichi IQ, de Paula Mariani BM, Frassetto FP, Siqueira SAC, de Castro Musolino NR, Cunha-Neto MBC, Ochman G, Cescato VAS, Machado MC, Trarbach EB, et al. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary. 2019;22(4):435–42.
-
Chaidarun SS, Swearingen B, Alexander JM. Differential expression of estrogen receptor-beta (ER beta) in human pituitary tumors: functional interactions with ER alpha and a tumor-specific splice variant. J Clin Endocrinol Metab. 1998;83(9):3308–15.
-
Oomizu S, Honda J, Takeuchi S, Kakeya T, Masui T, Takahashi S. Transforming growth factor-alpha stimulates proliferation of mammotrophs and corticotrophs in the mouse pituitary. J Endocrinol. 2000;165(2):493–501.
Acknowledgements
We thank all the participants enrolled in this study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Iran University of Medical Sciences No. IR.IUMS.REC.1398.082.
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the 1964 Helsinki Declaration, and was approved by the Ethics Committee of Iran University of Medical Sciences. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Cite this article
Hashemi-Madani, N., Cheraghi, S., Emami, Z. et al. Targeted analysis of Ubiquitin-Specific Peptidase (USP8) in a population of Iranian people with Cushing’s disease and a systematic review of the literature. BMC Endocr Disord 24, 86 (2024). https://doi.org/10.1186/s12902-024-01619-z
- Received
- Accepted
- Published
- DOIhttps://doi.org/10.1186/s12902-024-01619-z
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkProvided by the Springer Nature SharedIt content-sharing initiative
Keywords
- Ubiquitin-specific peptidase 8 (USP8)
- Ubiquitin-specific peptidase 48 (USP 48)
- Cushing’s disease
- Mutation
- Corticotroph adenoma
From https://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-024-01619-z
Filed under: Cushing's | Tagged: hypercortisolism, pituitary, transsphenoidal | Leave a comment »