-
Cushing H (1994) The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). 1932. Obes Res 2(5):486–508
Article CAS PubMed Google Scholar
-
Aranda G, Enseñat J, Mora M, Puig-Domingo M, Martínez de Osaba MJ, Casals G et al (2015) Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary 18(1):142–149
Article CAS PubMed Google Scholar
-
Pendharkar AV, Sussman ES, Ho AL, Hayden Gephart MG, Katznelson L (2015) Cushing’s disease: predicting long-term remission after surgical treatment. Neurosurg Focus 38(2):E13
Article PubMed Google Scholar
-
Etxabe J, Vazquez JA (1994) Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf) 40(4):479–484
Article CAS PubMed Google Scholar
-
Hameed N, Yedinak CG, Brzana J, Gultekin SH, Coppa ND, Dogan A et al (2013) Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16(4):452–458
Article CAS PubMed Google Scholar
-
Acebes JJ, Martino J, Masuet C, Montanya E, Soler J (2007) Early post-operative ACTH and cortisol as predictors of remission in Cushing’s disease. Acta Neurochir (Wien). ;149(5):471-7; discussion 7–9
-
Fernandez-Rodriguez E, Stewart PM, Cooper MS (2009) The pituitary-adrenal axis and body composition. Pituitary 12(2):105–115
Article CAS PubMed Google Scholar
-
van Haalen FM, Broersen LH, Jorgensen JO, Pereira AM, Dekkers OM (2015) Management of endocrine disease: mortality remains increased in Cushing’s disease despite biochemical remission: a systematic review and meta-analysis. Eur J Endocrinol 172(4):R143–R149
Article PubMed Google Scholar
-
Locatelli M, Vance ML, Laws ER (2005) Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 90(9):5478–5482
Article CAS PubMed Google Scholar
-
Blevins LS Jr., Christy JH, Khajavi M, Tindall GT (1998) Outcomes of therapy for Cushing’s disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab 83(1):63–67
CAS PubMed Google Scholar
-
Esposito F, Dusick JR, Cohan P, Moftakhar P, McArthur D, Wang C et al (2006) Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 91(1):7–13
Article CAS PubMed Google Scholar
-
Guo S, Wang Z, Kang X, Xin W, Li X (2021) A Meta-analysis of endoscopic vs. microscopic transsphenoidal surgery for non-functioning and functioning pituitary adenomas: comparisons of efficacy and safety. Front Neurol. ;12
-
Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C et al (2014) Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: a meta-analysis. World J Surg Oncol 12:94
Article PubMed PubMed Central Google Scholar
-
Yu SY, Du Q, Yao SY, Zhang KN, Wang J, Zhu Z, Jiang XB (2018) Outcomes of endoscopic and microscopic transsphenoidal surgery on non-functioning pituitary adenomas: a systematic review and meta-analysis. J Cell Mol Med 22(3):2023–2027
Article PubMed PubMed Central Google Scholar
-
Broersen LHA, van Haalen FM, Biermasz NR, Lobatto DJ, Verstegen MJT, van Furth WR et al (2019) Microscopic versus endoscopic transsphenoidal surgery in the Leiden cohort treated for Cushing’s disease: surgical outcome, mortality, and complications. Orphanet J Rare Dis 14(1):64
Article PubMed PubMed Central Google Scholar
-
Jho HD, Carrau RL (1997) Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg 87(1):44–51
Article CAS PubMed Google Scholar
-
Berker M, Işikay I, Berker D, Bayraktar M, Gürlek A (2014) Early promising results for the endoscopic surgical treatment of Cushing’s disease. Neurosurg Rev 37:105-114
-
Netea-Maier RT, van Lindert EJ, den Heijer M, van der Eerden A, Pieters GF, Sweep CG et al (2006) Transsphenoidal pituitary surgery via the endoscopic technique: results in 35 consecutive patients with Cushing’s disease. Eur J Endocrinol 154(5):675–684
Article CAS PubMed Google Scholar
-
Dehdashti AR, Gentili F (2007) Current state of the art in the diagnosis and surgical treatment of cushing disease: early experience with a purely endoscopic endonasal technique. Neurosurg Focus 23(3):E9
Article PubMed Google Scholar
-
Starke RM, Reames DL, Chen CJ, Laws ER, Jane JA (2013) Jr. Endoscopic transsphenoidal surgery for cushing disease: techniques, outcomes, and predictors of remission. Neurosurgery 72(2):240–247 discussion 7
Article PubMed Google Scholar
-
Sarkar S, Rajaratnam S, Chacko G, Mani S, Hesargatta AS, Chacko AG (2016) Pure endoscopic transsphenoidal surgery for functional pituitary adenomas: outcomes with Cushing’s disease. Acta Neurochir (Wien) 158(1):77–86 discussion
Article PubMed Google Scholar
-
Cebula H, Baussart B, Villa C, Assié G, Boulin A, Foubert L et al (2017) Efficacy of endoscopic endonasal transsphenoidal surgery for Cushing’s disease in 230 patients with positive and negative MRI. Acta Neurochir (Wien) 159(7):1227–1236
Article PubMed Google Scholar
-
Wagenmakers MA, Boogaarts HD, Roerink SH, Timmers HJ, Stikkelbroeck NM, Smit JW et al (2013) Endoscopic transsphenoidal pituitary surgery: a good and safe primary treatment option for Cushing’s disease, even in case of macroadenomas or invasive adenomas. Eur J Endocrinol 169(3):329–337
Article CAS PubMed Google Scholar
-
Esquenazi Y, Essayed WI, Singh H, Mauer E, Ahmed M, Christos PJ, Schwartz TH (2017) Endoscopic endonasal Versus Microscopic Transsphenoidal surgery for recurrent and/or residual pituitary adenomas. World Neurosurg 101:186–195
Article PubMed PubMed Central Google Scholar
-
Cavallo LM, Solari D, Tasiou A, Esposito F, de Angelis M, D’Enza AI, Cappabianca P (2013) Endoscopic endonasal transsphenoidal removal of recurrent and regrowing pituitary adenomas: experience on a 59-patient series. World Neurosurg 80(3–4):342–350
Article PubMed Google Scholar
-
Perlman JE, Johnston PC, Hui F et al (2021) Pitfalls in performing and interpreting Inferior Petrosal Sinus Sampling: personal experience and literature review. J Clin Endocrinol Metab 106(5):e1953–e1967. https://doi.org/10.1210/clinem/dgab012
Article PubMed PubMed Central Google Scholar
-
Yogi-Morren D, Habra MA, Faiman C et al (2015) Pituitary MRI findings in patients with pituitary and ectopic ACTH-dependent cushing syndrome: does a 6-mm pituitary tumor size cut-off value exclude ectopic ACTH syndrome? Endocr Pract 21(10):1098–1103
Article Google Scholar
-
Negm HM, Al-Mahfoudh R, Pai M, Singh H, Cohen S, Dhandapani S et al (2017) Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J Neurosurg 127(2):397–408
Article PubMed Google Scholar
-
Cooke RS, Jones RA (1994) Experience with the direct transnasal transsphenoidal approach to the pituitary fossa. Br J Neurosurg 8(2):193–196
Article CAS PubMed Google Scholar
-
Chee GH, Mathias DB, James RA, Kendall-Taylor P (2001) Transsphenoidal pituitary surgery in Cushing’s disease: can we predict outcome? Clin Endocrinol (Oxf) 54(5):617–626
Article CAS PubMed Google Scholar
-
Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B (2005) Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 63(5):549–559
Article PubMed Google Scholar
-
Yap LB, Turner HE, Adams CB, Wass JA (2002) Undetectable postoperative cortisol does not always predict long-term remission in Cushing’s disease: a single centre audit. Clin Endocrinol (Oxf) 56(1):25–31
Article CAS PubMed Google Scholar
-
Liu X, Dai C, Bao X, Deng K, Yao Y, Sun B et al (2021) Treatment and outcomes of recurrent/persistent Cushing’s disease: a single-center experience. Ann Palliat Med 10(3):2494–2504
Article PubMed Google Scholar
-
Valderrábano P, Aller J, García-Valdecasas L, García-Uría J, Martín L, Palacios N, Estrada J (2014) Results of repeated transsphenoidal surgery in Cushing’s disease. Long-term follow-up. Endocrinol Nutr 61(4):176–183
Article PubMed Google Scholar
-
Wang B, Zheng S, Ren J, Zhong Z, Jiang H, Sun Q et al (2022) Reoperation for Recurrent and Persistent Cushing’s Disease without Visible MRI Findings. J Clin Med 11(22): 6848
-
Burke WT, Penn DL, Repetti CS, Iuliano S, Laws ERJ (2019) Outcomes after repeat transsphenoidal surgery for recurrent Cushing Disease: updated. Neurosurgery 85(6):E1030–E6
Article PubMed Google Scholar
-
Bakiri F, Tatai S, Aouali R, Semrouni M, Derome P, Chitour F, Benmiloud M (1996) Treatment of Cushing’s disease by transsphenoidal, pituitary microsurgery: prognosis factors and long-term follow-up. J Endocrinol Invest 19(9):572–580
Article CAS PubMed Google Scholar
-
Dickerman RD, Oldfield EH (2002) Basis of persistent and recurrent cushing disease: an analysis of findings at repeated pituitary surgery. J Neurosurg 97(6):1343–1349
Article PubMed Google Scholar
-
Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, Laws ER (2008) Jr. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93(2):358–362
Article CAS PubMed Google Scholar
-
Castinetti F, Brue T, Ragnarsson O (2019) Radiotherapy as a tool for the treatment of Cushing’s disease. Eur J Endocrinol 180(5):D9–d18
Article PubMed Google Scholar
-
Tajudeen BA, Mundi J, Suh JD, Bergsneider M, Wang MB (2015) Endoscopic endonasal surgery for recurrent pituitary tumors: technical challenges to the surgical approach. J Neurol Surg B Skull Base 76(1):50–56
Article PubMed Google Scholar
-
Hwang JM, Kim YH, Kim JW, Kim DG, Jung HW, Chung YS (2013) Feasibility of endoscopic endonasal approach for recurrent pituitary adenomas after microscopic trans-sphenoidal approach. J Korean Neurosurg Soc 54(4):317–322
Article PubMed PubMed Central Google Scholar
-
Berker M, Hazer DB, Yücel T, Gürlek A, Cila A, Aldur M, Onerci M (2012) Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15(3):288–300
Article PubMed Google Scholar
-
Rudnik A, Zawadzki T, Wojtacha M, Bazowski P, Zubgałuszka-Ignasiak B, Duda I (2005) [Endoscopic transsphenoidal treatment of pituitary adenomas]. Neurol Neurochir Pol 39(1):17–23 discussion 4–5
PubMed Google Scholar
-
Minniti G, Osti M, Jaffrain-Rea ML, Esposito V, Cantore G, Maurizi Enrici R (2007) Long-term follow-up results of postoperative radiation therapy for Cushing’s disease. J Neurooncol 84(1):79–84
Article PubMed Google Scholar
-
Estrada J, Boronat M, Mielgo M, Magallón R, Millan I, Díez S et al (1997) The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med 336(3):172–177
Article CAS PubMed Google Scholar
-
Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Sutton ML (1990) Long-term follow-up of low-dose external pituitary irradiation for Cushing’s disease. Clin Endocrinol (Oxf) 33(4):445–455
Article CAS PubMed Google Scholar



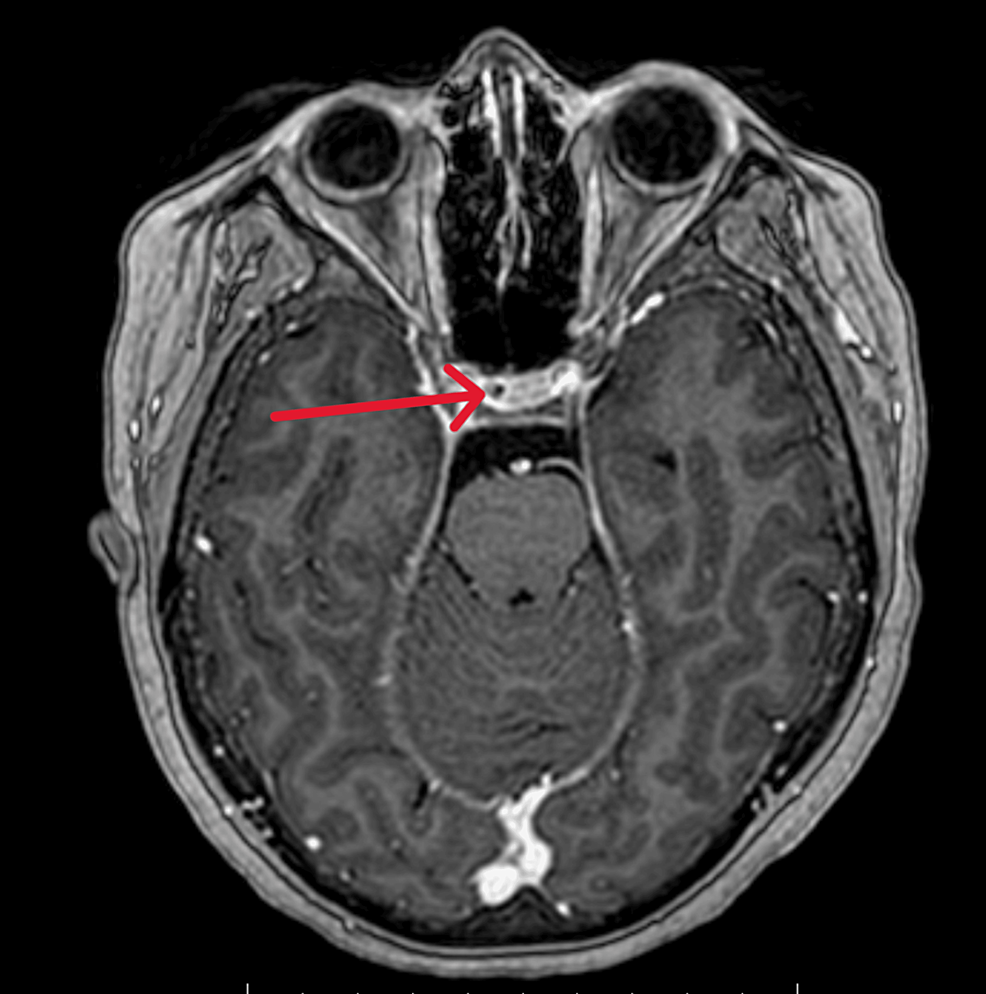
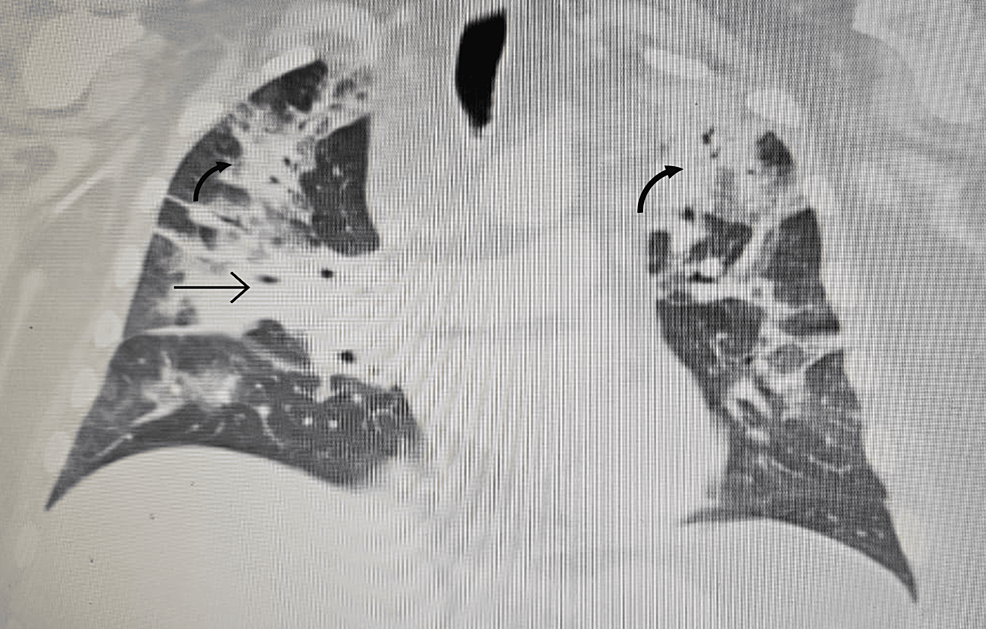
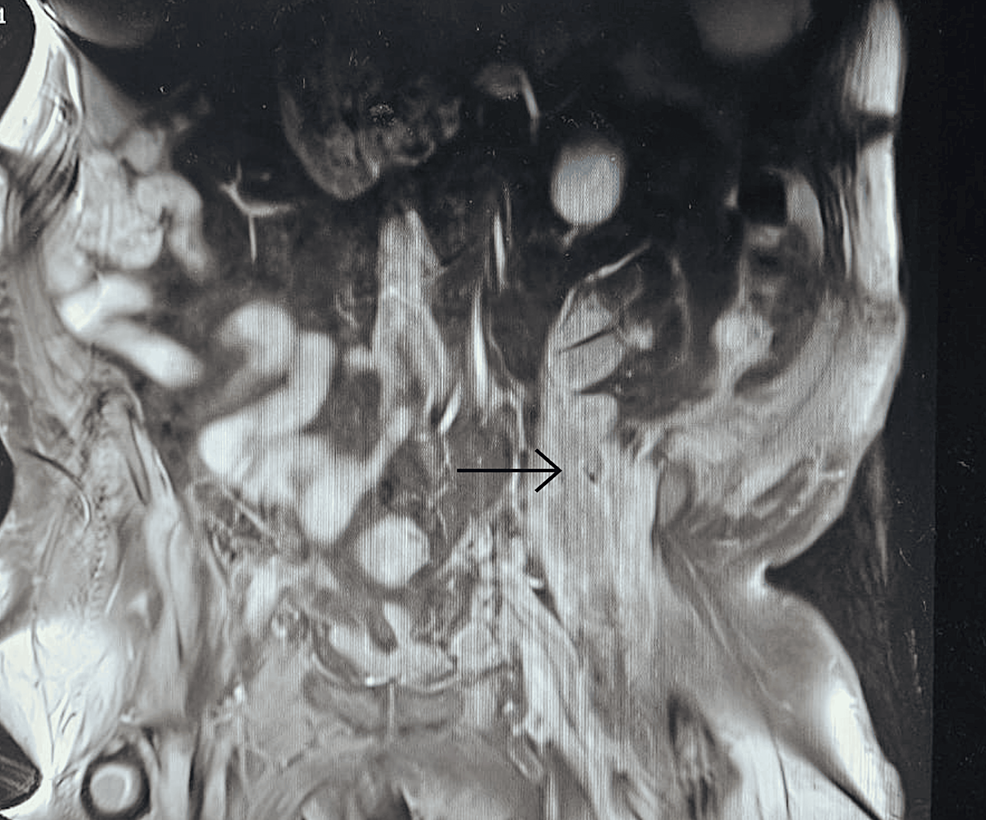
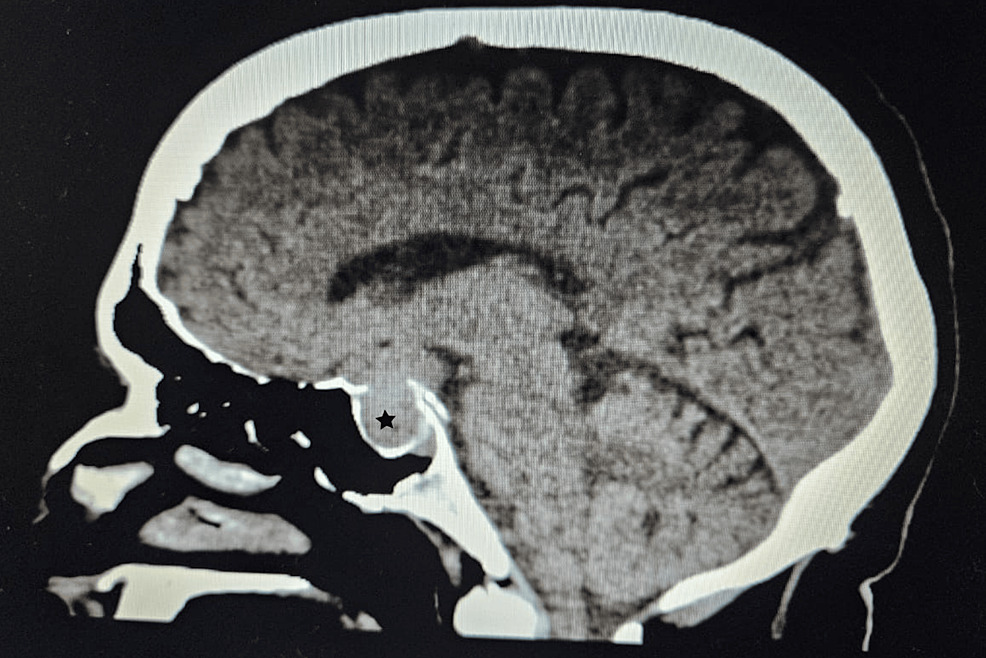

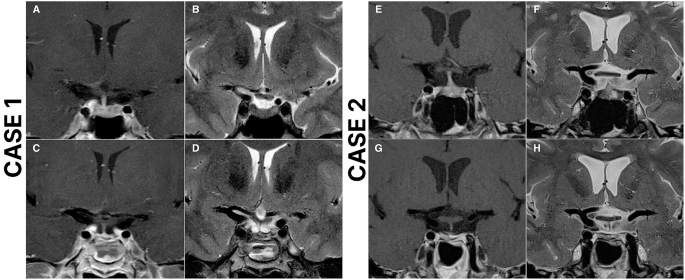
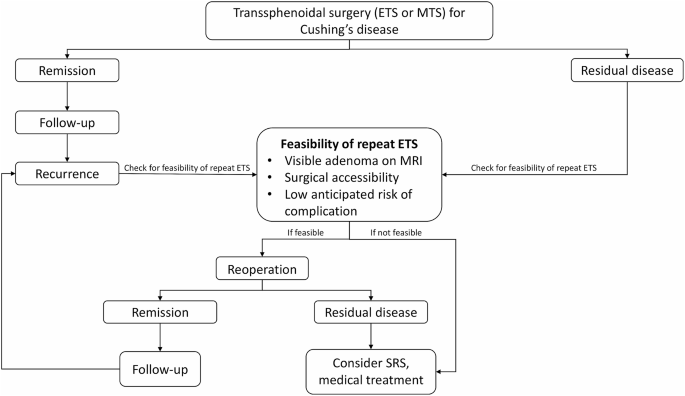
From https://www.cureus.com/articles/243881-unveiling-the-uncommon-cushings-syndrome-cs-masquerading-as-severe-hypokalemia#!/