Introduction
Methods
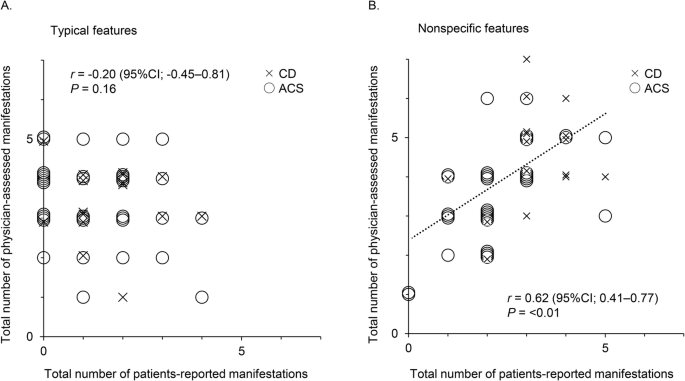
Clinical presentation of CS in children
|
Magiakou 1994 [23]
|
Devoe 1997 [25]
|
Storr 2011 [26]
|
Shah 2011 [22]
|
Lonser 2013 [27]
|
Guemes 2016 [28]
|
|||
|---|---|---|---|---|---|---|---|---|
|
Number of patients (F/M)
|
59 (37/22)
|
42 (25/17)
|
41 (15/26)
|
48 (19/29)
|
200 (106/94)
|
30 (14/16)
|
||
|
Period of observation
|
1982–1992
|
1974–1993
|
1983–2010
|
1988–2008
|
1982–2010
|
1983–2013
|
||
|
Subtype of CS
|
Pituitary (50)
|
Adrenal (6)
|
Ectopic (3)
|
Pituitary
|
Pituitary
|
Pituitary
|
Pituitary
|
Pituitary (16), Adrenal (11), Ectopic (2), Unknown (1)
|
|
Mean age at onset (y) or duration of symptoms (m)
|
11±4 y
|
9±6 y
|
10±3 y
|
NA
|
NA
|
23.6 ± 14.2 m
|
10.6 ± 3.6 y
|
12 m (6–18)
|
|
Mean age at diagnosis (range)
|
14±4
|
10±5
|
11±4
|
13.1 y (6.5–18)a
|
12.3 ± 3.5 y (5.7–17.8)
|
14.85 ± 2.5 y (9–19)
|
13.7 ± 3.7 y
|
8.9 (0.2–15.5)a
|
|
SDS Height at diagnosis (range)
|
-1.3±1.5
|
-1.0±1.3
|
-0.1±0.9
|
-1.8 (-3.5 to + 0.3)
|
-1.8 ± 1.3 (-1.2 to -4.2)
|
NAb
|
NA
|
-0.3 (-3.2 to + 3.0)c
|
|
Signs and symptoms (%)
|
||||||||
|
Weight gain
|
90
|
92
|
98
|
98
|
93
|
76.6
|
||
|
Growth retardation
|
83
|
84
|
100
|
83
|
63
|
36.6
|
||
|
Facial changes
|
46
|
100
|
98
|
63
|
||||
|
Fatigue
|
44
|
67
|
61
|
48
|
40
|
|||
|
Pubertal lack or delay
|
60
|
10
|
||||||
|
Hirsutism
|
78
|
46
|
59
|
56
|
56.6
|
|||
|
Acne
|
47
|
46
|
44
|
47
|
50
|
|||
|
Amenorrhea (primary or secondary
|
78
|
49
|
||||||
|
Virilization
|
38
|
76
|
26.6
|
|||||
|
Gynecomastia
|
16
|
|||||||
|
Osteopenia
|
74
|
|||||||
|
Dorsal cervical fat pad
|
28
|
69
|
||||||
|
Striae rubrae
|
61
|
36
|
49
|
58
|
55
|
26.6
|
||
|
Acanthosis nigricans
|
12
|
75
|
32
|
|||||
|
Headache
|
26
|
51
|
38
|
|||||
|
Hypertension
|
47
|
63
|
49
|
71
|
36
|
50
|
||
|
Psychiatric disorders
|
19
|
44
|
59
|
46
|
31
|
43.3
|
||
|
Sleep disturbances
|
20
|
|||||||
|
Muscle weakness
|
48
|
|||||||
|
Easy bruising
|
28
|
17
|
25
|
20
|
||||
|
Glucose intolerance or diabetes
|
25
|
7
|
||||||
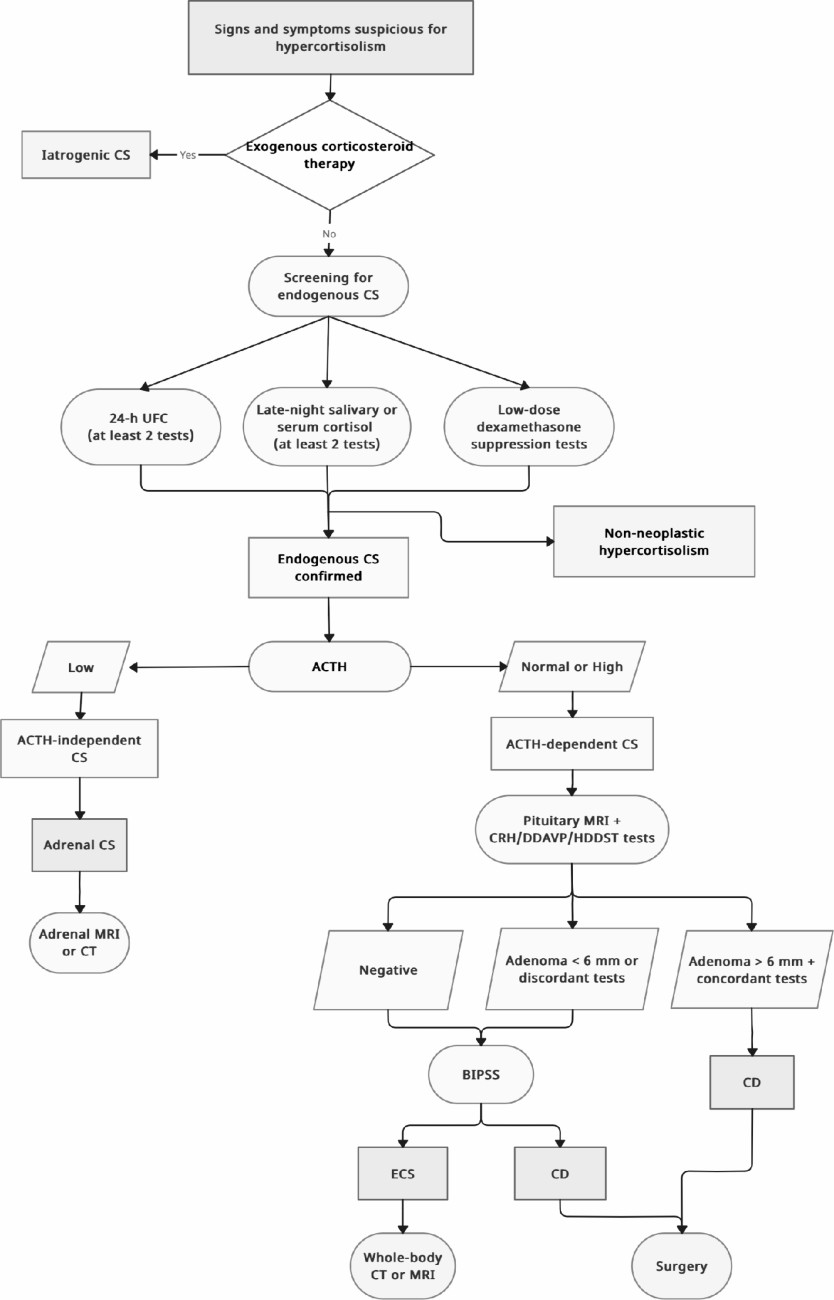
Diagnostic workup for CS
Screening for endogenous hypercortisolism
|
Author
|
Population Age (mean)
|
Subject characteristics (N)
|
Test
|
Cut-off
|
Sensibility
|
Specificity
|
|---|---|---|---|---|---|---|
|
Bickler 1994 [54]
|
15.7 y (pituitary)
8.1 y (adrenal)
|
Pituitary (10)
Adrenal (2)
|
UFC
|
> 60 mg/m2
|
100% (8/8)
|
|
|
LDDST
|
< 50% of basal serum cortisol
|
91% (10/11)
|
||||
|
Devoe 1997 [25]
|
13.1 y (6.5–18)a
|
Pituitary (42)
|
UFC
|
> 70 µg/m2
|
86% (25/29)
|
|
|
Martinelli 1999 [49]
|
10.2 ± 5 y
|
Pituitary (5), Adrenal (6), Obese controls (21)
|
Late-night salivary cortisol
|
> 7.5 nmol/l
|
100% (11/11)
|
95.2% (20/21)
|
|
Gafni 2000 [39]
|
5–17 y
|
CS patients (14), Healthy controls (53)
|
UFC
|
> 72 µg/m2
|
93% (13/14)
|
100% (53/53)
|
|
Late-night salivary cortisol
|
> 7.5 nmol/l
|
93% (13/14)
|
100% (53/53)
|
|||
|
Davies 2005 [47]
|
12.2 y
|
Pituitary (14)
|
Late-night serum cortisol
|
> 50 nmol/l [1.8 µg/dl]
|
100% (14/14)
|
|
|
Batista 2007 [38]
|
3–18 y
|
Pituitary (80), Adrenal (25), Controls (20)
|
UFC
|
> 70 µg/m2
|
88% (92/105) [PPV 98%]
|
90% (18/20) [NPV 58%]
|
|
Late-night serum cortisol
|
> 4.4 µg/dl
|
99% (104/105)
[PPV 100%]
|
100% (20/20) [NPV 95%]
|
|||
|
Shah 2011 [22]
|
14.85 ± 2.5 y
|
Pituitary (48)
|
Late-night serum cortisol
|
> 3.2 µg/dl
|
100% (38/38)
|
|
|
LDDST (30 µg/kg/day [max 2 mg/day] divided every 6 h for 48 h
|
≥ 1.8 µg/dl
|
100% (48/48)
|
||||
|
≥ 5 µg/dl
|
94% (45/48)
|
|||||
|
Storr 2011 [26]
|
12.3 ± 3.5 y
|
Pituitary (41)
|
LDDST (30 µg/kg/day [max 2 mg/day] divided every 6 h for 48 h)
|
< 50 nmol/l [1.8 µg/dl]
|
92% (35/38)
|
|
|
Lonser 2013 [27]
|
13.7 ± 3.7 y
|
Pituitary (200)
|
UFC
|
Age-appropriate reference
|
99% (177/179)
|
|
|
> 70 µg/m2
|
88% (155/177)
|
|||||
|
Late-night serum cortisol
|
> 7.5 µg/dl
|
97% (188/193)
|
||||
|
Shapiro 2016 [40]
|
11.7 y (pituitary), 12.9 y (adrenal), 11.5 y (controls)
|
Pituitary (39), Adrenal (8), Control (19)
|
UFC (different assays)
|
Corrected for BSA
|
89% (34/38)
|
100%
|
|
Wędrychowicz 2019 [55]
|
11.7 y
|
Pituitary (4)
|
UFC
|
> 55 µg/24 h
|
100% (4/4)
|
|
|
Late-night serum cortisol
|
> 4.4 µg/dl
|
100% (4/4)
|
||||
|
Overnight DST (1 mg at 11.00 p.m.)
|
< 1.8 µg/dl
|
75% (3/4)
|
||||
|
Guemes 2016 [28]
|
8.9 y (0.2–15.5)a
|
Pituitary (16), Adrenal (11), Ectopic (2), Unknown (1)
|
UFC
|
> 275 nmol [100 µg]/24 h
|
94% (17/18)
|
|
|
Late-night serum cortisol
|
> 138 nmol/l [5 µg/dl]
|
100% (27/27)
|
||||
|
LDDST (20 µg/kg/day [max 2 mg/day] divided every 6 h for 48 h)
|
< 50 nmol/l [1.8 µg/dl]
|
100% (20/20)
|
24-h Urinary free cortisol (UFC)
Late night cortisol
Low-dose dexamethasone suppression tests (DST)
Etiological diagnosis of endogenous CS
Basal electrolytes and ACTH
CRH stimulation test
Desmopressin test
Standard high dose dexamethasone suppression test (HDDST)
Imaging
Pituitary magnetic resonance imaging (MRI)
Bilateral petrosal sinus sampling (BIPSS)
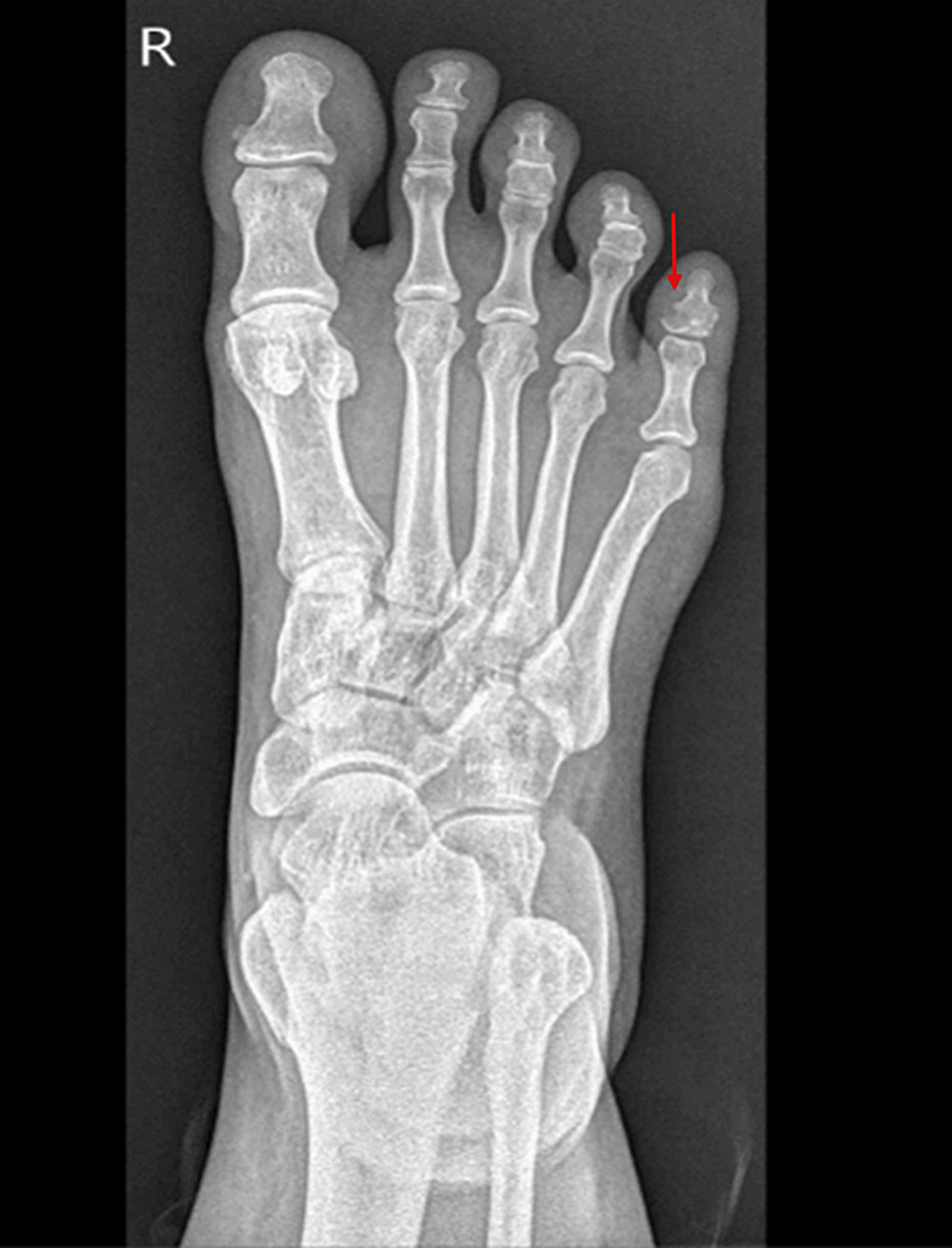
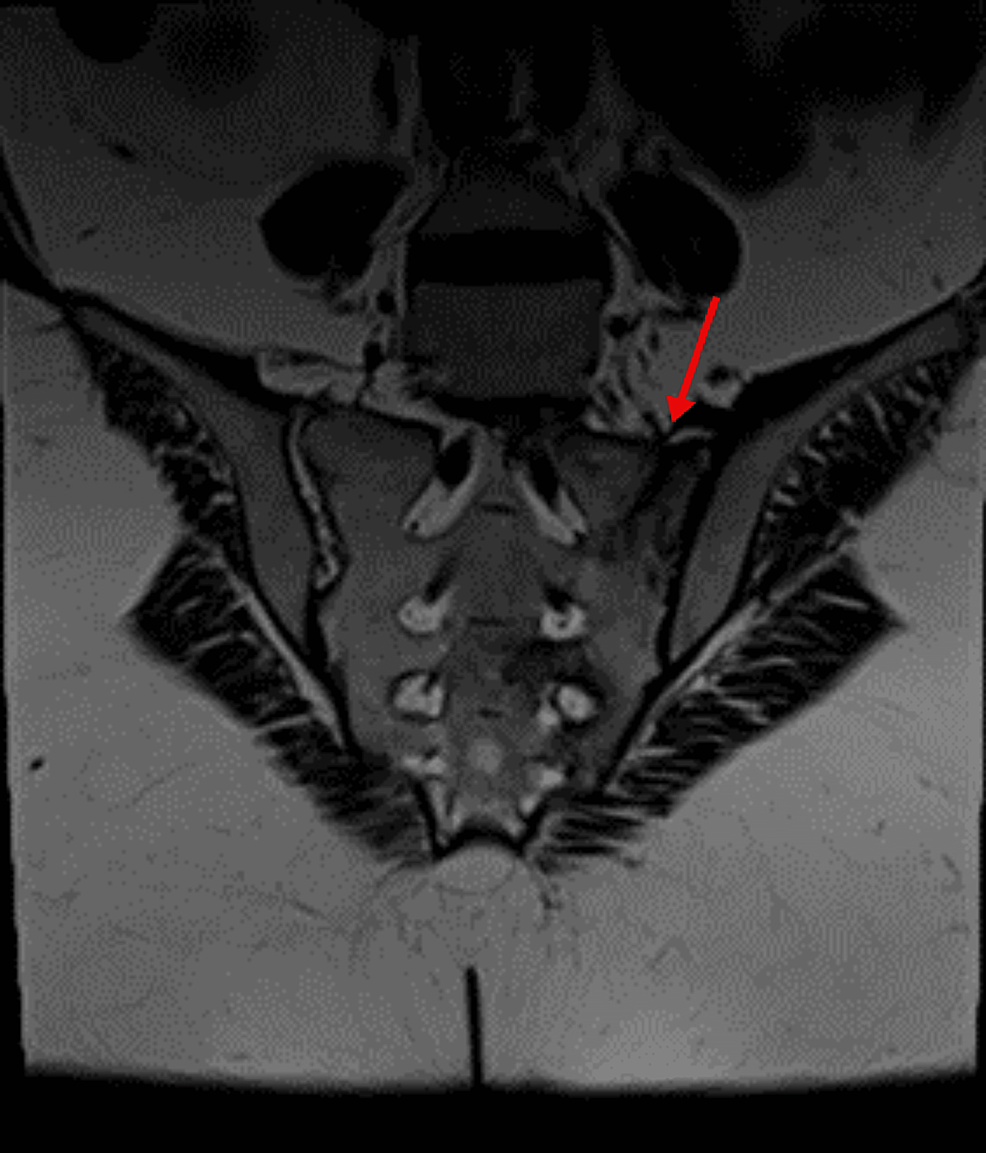
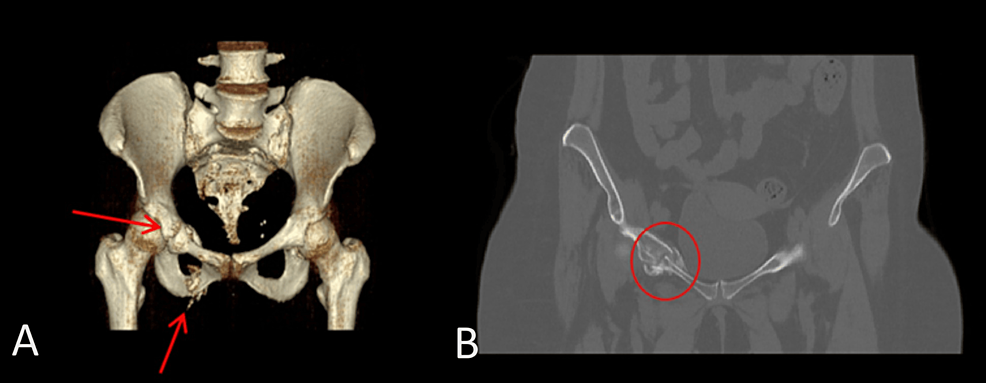
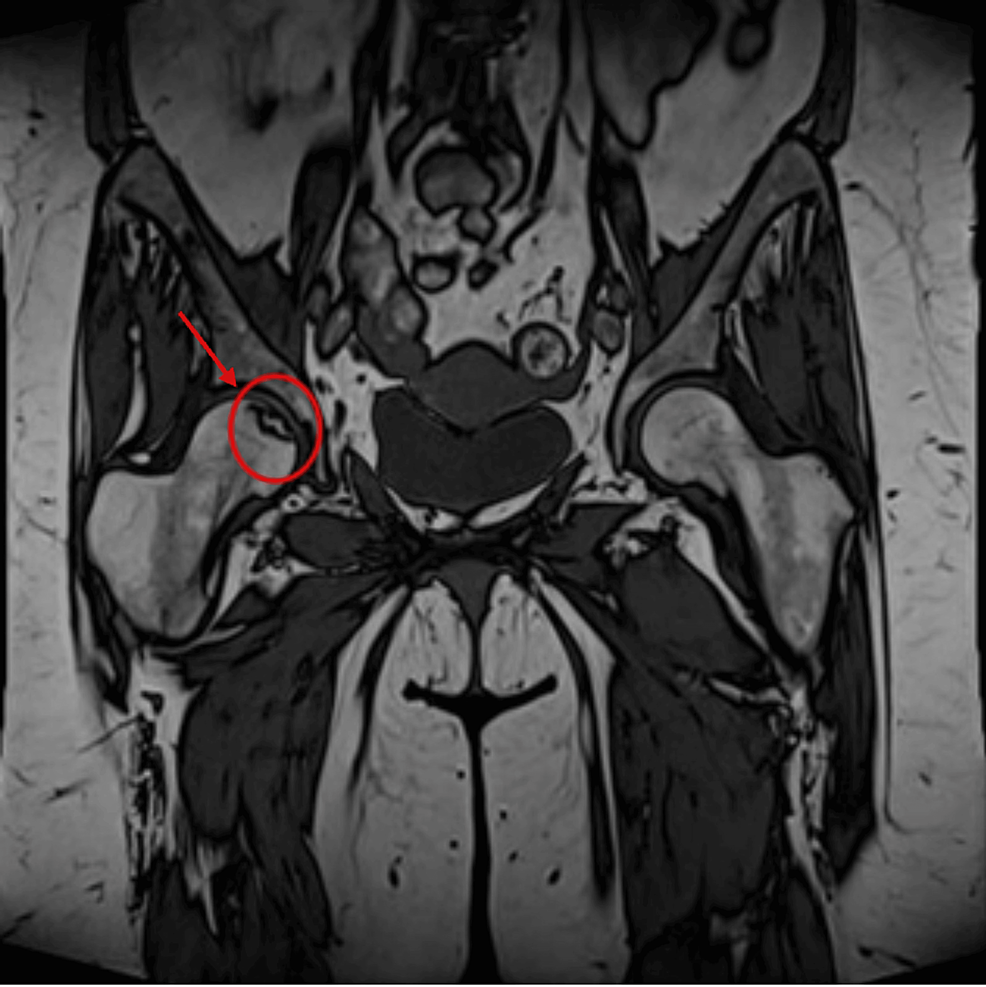
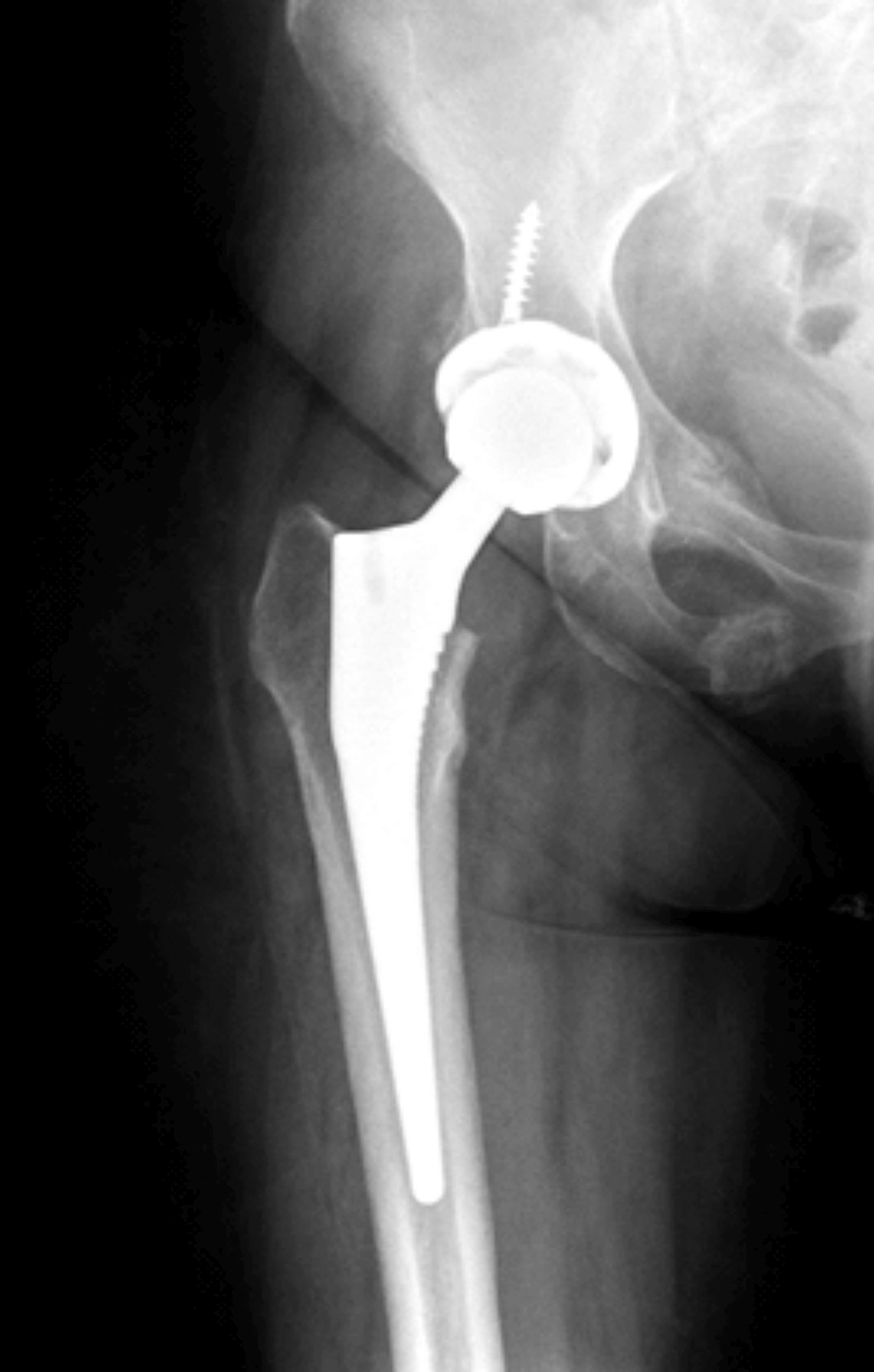
Radiological anatomic imaging
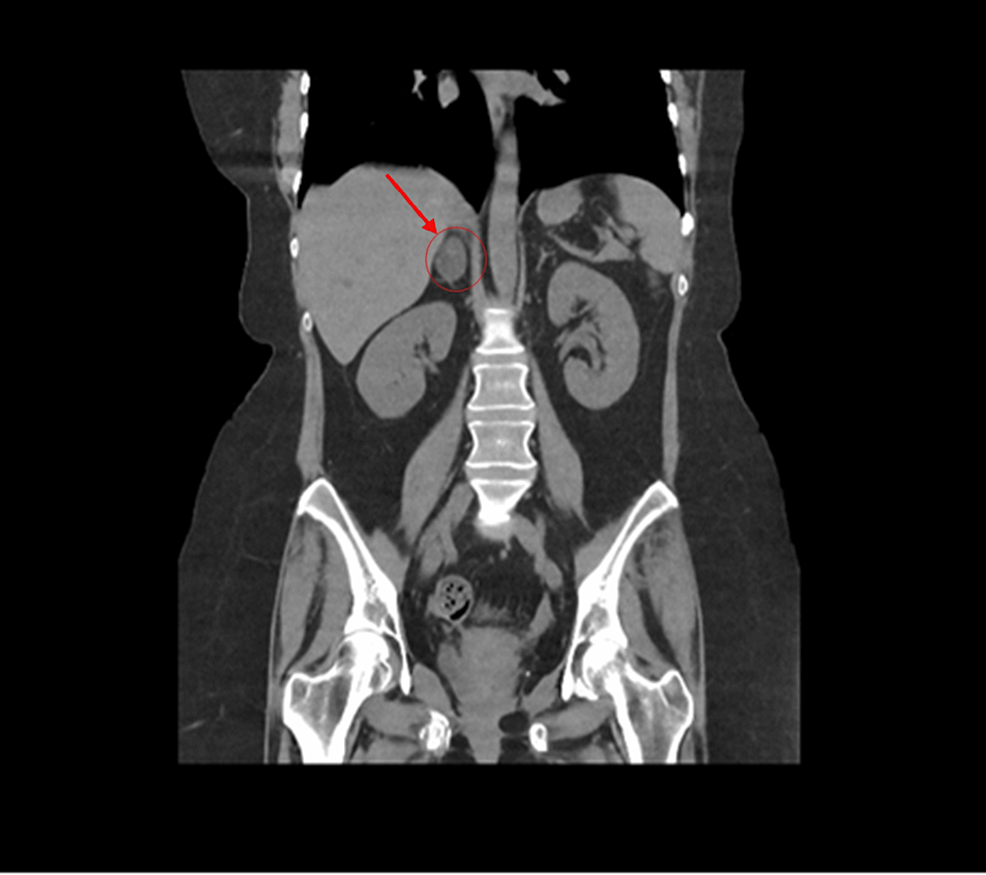
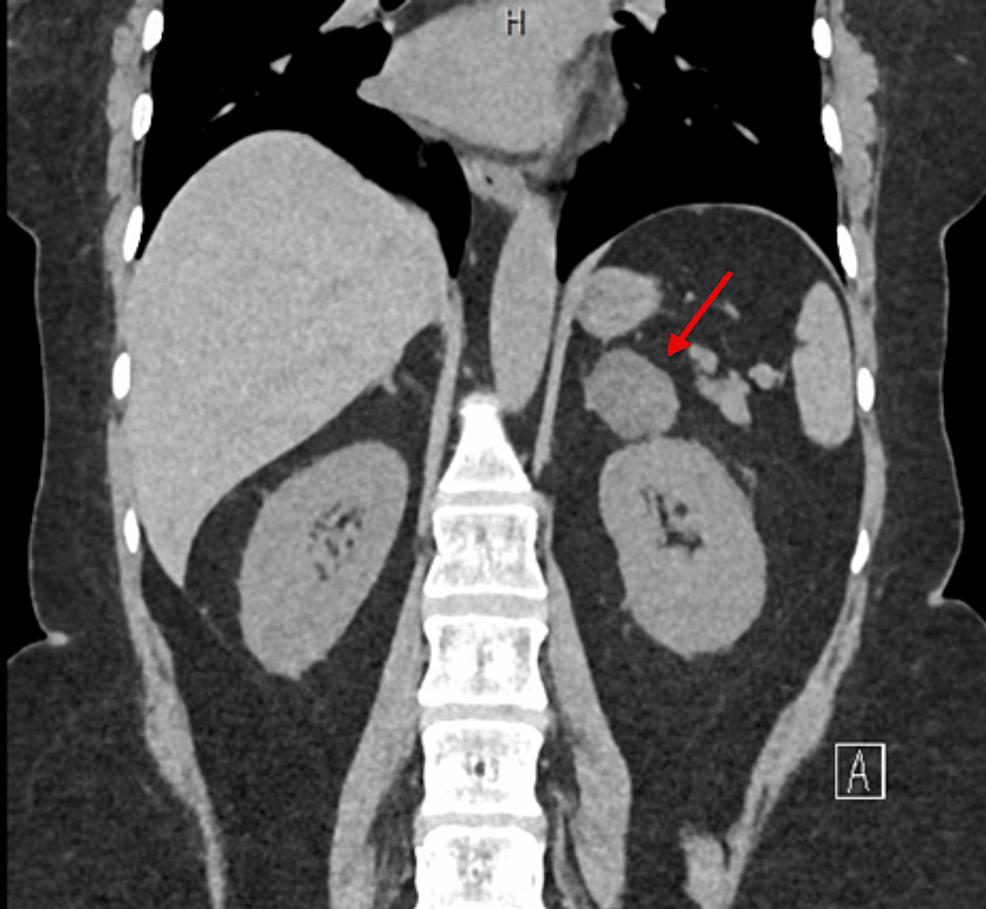
Functional imaging
Algorithm approach
Conclusions
Declarations
Ethical approval
Informed consent
Conflict of interest
Publisher’s note
Filed under: Cushing's | Tagged: children, Cushing's, pediatric | Leave a comment »



 View Full Size
View Full Size View Full Size
View Full Size