Cushing’s syndrome—also referred to as hypercortisolism—is fairly rare. However, researchers have boiled down a few key causes of Cushing’s syndrome, which you’ll read about below.
The cause of Cushing’s syndrome boils down to: Your body is exposed to too much cortisol. There are a few ways that this over-exposure can happen, including taking certain medications and having a tumor on your pituitary gland or adrenal gland.
Can Taking Corticosteroids Cause Cushing’s Disease?
One particular type of medication can cause Cushing’s syndrome: corticosteroids. But rest assured: Not all steroid medications cause Cushing’s syndrome. It’s more common to develop Cushing’s syndrome from steroids you take in pill form or steroids you inject. Steroid creams and steroids you inhale are not common causes of Cushing’s syndrome.
Some steroid medications have the same effect as the hormone cortisol does when produced in your body. But as with an excessive production of cortisol in your body, taking too much corticosteroid medications can, over time, lead to Cushing’s syndrome.
It’s common for people with asthma, rheumatoid arthritis, and lupus to take corticosteroids. Prednisone (eg, Deltasone) is an example of a corticosteroid medication.
Other Cushing’s Disease Causes
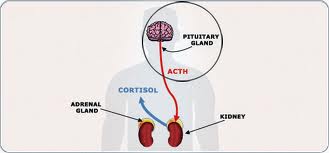
Your body can over-produce cortisol or adrenocorticotropic hormone (ACTH). The pituitary gland secretes ACTH, which is in charge of stimulating the adrenal glands to produce cortisol, and the adrenal glands are responsible for releasing cortisol into the bloodstream.
Cortisol performs important tasks in your body, such as helping to maintain blood pressure and regulate how your body metabolizes proteins, fats, and carbohydrates, so it’s necessary for your body to maintain normal levels of it.
The following can cause excessive production of cortisol or ACTH, leading to Cushing’s syndrome.
- Pituitary gland tumors: A benign (non-cancerous) tumor of the pituitary gland can secrete an excess amount of ACTH, which can cause Cushing’s syndrome. Also known as pituitary adenomas, benign tumors of the pituitary gland affect women 5 times more often than men.
- Adrenal gland tumors: A tumor in one of your adrenal glands can lead to Cushing’s syndrome by causing too much cortisol to enter your bloodstream. Most of these tumors are non-cancerous (called adrenal adenomas).
Cancerous adrenal tumors—called adrenocortical carcinomas—are relatively rare. These types of tumors typically cause extremely high levels of cortisol and very rapid development of symptoms.
- Other tumors in the body: Certain tumors that develop outside the pituitary gland can also produce ACTH. When this happens, it’s known as ectopic ACTH syndrome. Ectopic means that something is in an abnormal place or position. In this case, only the pituitary gland should produce ACTH, so if there is a tumor producing ACTH and it isn’t located on the pituitary, it’s ectopic.
It’s unusual to have a tumor that secretes ACTH outside the pituitary. These tumors are usually found in the pancreas, lungs, or thyroid, and they can be benign or malignant (cancerous).
The most common forms of ACTH-producing tumors are small cell lung cancer, which accounts for about 13% of all lung cancer cases, and carcinoid tumors—small, slow-growing tumors that arise from hormone-producing cells in various parts of the body.
- Familial Cushing’s syndrome: Although it’s rare, Cushing’s syndrome can develop from an inherited tendency to have tumors on one or more of your endocrine glands. Some inherited conditions, such as multiple endocrine neoplasia (MEN 1), can involve tumors that over-produce cortisol or ACTH, leading to Cushing’s syndrome.
If you think you could have Cushing’s syndrome or you have questions about the causes of Cushing’s syndrome, talk to your doctor immediately.
Written by Julie M. Gentile | Reviewed by Daniel J. Toft MD, PhD, adapted from http://www.endocrineweb.com/conditions/cushings-syndrome/cushings-syndrome-causes
Filed under: adrenal, Cushing's, pituitary | Tagged: ACTH, adrenal, corticosteroids, Cushing's Syndrome, familial, hormones, hypercortisolism, pituitary, prednisone, steroids, tumor | 1 Comment »