Abstract
Introduction
Cushing’s syndrome, or hypercortisolism, occurs after prolonged exposure to excess cortisol, and can be characterized by moon facies, central fat redistribution, proximal limb muscle weakness and wasting, and abdominal striae. Medical literature points to a relationship between hypercortisolism and hypercoagulability, with higher rates of venous thromboembolism noted. Current guidelines recommend prophylaxis with low-molecular weight heparin (LMWH), but there is little evidence to support LMWH over other forms of anticoagulation.
Methods
We utilized TriNetX US Collaborative Network (TriNetX, LLC, Cambridge, Massachusetts, United States) to investigate the efficacy of different forms of anticoagulation in patients with hypercortisolism, defined by International Classification of Diseases, Tenth Revision (ICD-10) codes. Adult patients with hypercortisolism and prescribed enoxaparin, a form of LMWH, were compared to patients with hypercortisolism prescribed unfractionated heparin, warfarin, apixaban, and aspirin at 81 mg. Groups were propensity-matched according to age at index event, sex, race, ethnicity, and comorbid conditions. The outcomes studied included pulmonary embolism (PE), upper extremity deep vein thrombosis (UE DVT), lower extremity deep venous thrombosis (LE DVT), superficial venous thrombosis (superficial VT), bleeding, transfusion, and all-cause mortality.
Results
No significant differences in outcomes were noted between enoxaparin and heparin, warfarin, or apixaban in patients with hypercortisolism of any cause. Uniquely, the enoxaparin cohort had significantly higher risk of PE, LE DVT, and all-cause mortality compared to the aspirin 81 mg cohort (PE: hazard ratio (HR) 1.697, 95%CI 1.444-1.994, p=0.0345; LE DVT: HR 1.492, 95%CI 1.28-1.738, p=0.0017; mortality: HR 1.272, 95%CI 1.167-1.386, p=0.0002). With further sub-analysis of pituitary-dependent (Cushing’s Disease), enoxaparin continued to demonstrate a higher risk for LE DVT (HR 1.677, 95%CI 1.353-2.079, p=0.0081), and all-cause mortality (HR 1.597, 95%CI 1.422-1.794, p=0.0005).
Conclusion
Although LMWH is currently recommended as the gold standard for anticoagulation in patients with hypercortisolism, our evidence suggests that low-dose antiplatelets such as aspirin 81 mg could outperform it. Further research is warranted to confirm and replicate our findings.
Introduction
Cortisol is produced within the zona fasciculata of the adrenal cortex and is typically released under stress [1]. Cushing’s Syndrome, first defined in 1912 by American neurosurgeon Harvey Cushing, is a state of prolonged hypercortisolism, presenting with classic phenotypic manifestations, including moon facies, central fat deposition, proximal limb muscle weakness and muscle wasting, and abdominal striae [2]. Cushing’s syndrome can be exogenous (medication-induced/iatrogenic) or endogenous (ectopic adrenocorticotrophic hormone (ACTH), pituitary-dependent, or adrenal adenoma/carcinoma) [3]. Pituitary adenomas causing ACTH-dependent cortisol excess account for 80% of endogenous cases of Cushing’s Syndrome and are more specifically termed Cushing’s Disease [4]. Overall, however, the most common cause of Cushing’s Syndrome is iatrogenic, from exogenous corticosteroid administration [5].
Hypercortisolism has also been demonstrated to affect coagulation, though the mechanism is unclear [6]. Both venous thromboemboli and pulmonary emboli rates are increased among these patients [7]. The Endocrine Society Guidelines for Treatment of Cushing Syndrome describe altered coagulation profiles that take up to one year to normalize [8]. As a result, limited guidelines recommend prophylactic anticoagulation in Cushing syndrome; while low-molecular-weight heparin (LMWH) is the gold standard, there is little evidence behind this recommendation [9]. Furthermore, few studies assessed individual Cushing’s Syndrome subtypes and associated clotting risks or anticoagulation impact. It is currently unknown whether the antagonistic effects of cortisol will be augmented or hindered by anticoagulation other than LMWH.
This retrospective multicenter study aimed to address this paucity in data by analyzing differences among various forms of anticoagulation. Patients with Cushing syndrome who were on one of three common anticoagulants, or aspirin, were compared to patients with Cushing’s Syndrome on enoxaparin, an LMWH considered the gold standard for prophylaxis in this population. Primary objectives included end-points concerning thromboses (such as pulmonary embolism (PE), upper and lower extremity deep vein thromboses (DVTs), and superficial venous thrombosis (VT)). Secondary objectives included analyzing safety profiles (bleeding, transfusion requirements, and all-cause mortality).
Materials & Methods
Eligibility criteria
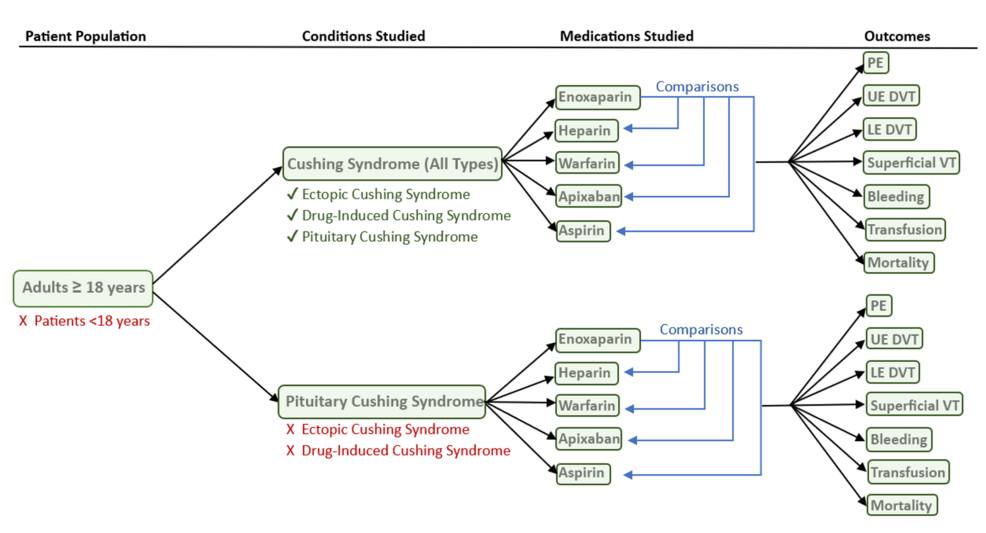
TriNetX Global Collaborative network (TriNetX, LLC, Cambridge, Massachusetts, United States), a nationwide database of de-identified health data across multiple large healthcare organizations (HCOs), was utilized to compile patients according to International Classification of Diseases, Tenth Revision (ICD-10) codes (Figure 1).
Figure 1: Flow chart for inclusion and exclusion criteria for the study
PE: pulmonary embolism; VT: venous thrombosis; DVT: deep vein thrombosis; UE: upper extremity; LE: lower extremity
ICD-10 codes included those related to Cushing’s Syndrome and one of five studied medications: enoxaparin, heparin, apixaban, warfarin, and aspirin, included in Tables 1 and 2, respectively. ICD-10 codes also included those related to outcomes, including PE, upper extremity (UE) DVT, lower extremity (LE) DVT, superficial VT, bleeding, transfusion, and all-cause mortality (Table 3). Measures of association involved calculating risk differences and relative risks (RRs) with 95% confidence intervals (CIs) to compare the proportion of patients experiencing each outcome across cohorts.
| Cushing’s Syndrome Type | ICD-10 Code |
| Cushing Syndrome (unspecified) | Drug-Induced Cushing Syndrome (UMLS:ICD10CM:E24.2) |
| Other Cushing Syndrome (UMLS:ICD10CM:E24.8) | |
| Cushing Syndrome, Unspecified (UMLS:ICD10CM:E24.9) | |
| Pituitary-Dependent Cushing Disease (UMLS:ICD10CM:E24.0) | |
| Cushing Syndrome (UMLS:ICD10CM:E24) | |
| Ectopic ACTH Syndrome (UMLS:ICD10CM:E24.3) | |
| Cushing Syndrome (pituitary) | Pituitary-Dependent Cushing Disease (UMLS:ICD10CM:E24.0 ) |
Table 1: International Classification of Disease (ICD)-10 codes utilized to identify patients with Cushing Syndrome in the TriNetX database
| Medication | ICD-10 Code |
| Enoxaparin | NLM:RXNORM:67108 |
| Warfarin | NLM:RXNORM:11289 |
| Heparin | NLM:RXNORM:5224 |
| Apixaban | NLM:RXNORM:1364430 |
| Aspirin | NLM:RXNORM:1191 |
Table 2: International Classification of Disease (ICD)-10 codes utilized to identify anticoagulants and antiplatelets studied in the TriNetX database
| Outcome | ICD-10 Codes | ||
| Pulmonary Embolism | Pulmonary Embolism | UMLS:ICD10CM:I26 | |
| Upper Extremity DVT | Acute embolism and thrombosis of deep veins of unspecified upper extremity | UMLS:ICD10CM:I82.629 | |
| Chronic embolism and thrombosis of deep veins of unspecified upper extremity | UMLS:ICD10CM:I82.729 | ||
| Acute embolism and thrombosis of deep veins of right upper extremity | UMLS:ICD10CM:I82.621 | ||
| Acute embolism and thrombosis of deep veins of left upper extremity | UMLS:ICD10CM:I82.622 | ||
| Acute embolism and thrombosis of deep veins of upper extremity, bilateral | UMLS:ICD10CM:I82.623 | ||
| Chronic embolism and thrombosis of deep veins of right upper extremity | UMLS:ICD10CM:I82.721 | ||
| Chronic embolism and thrombosis of deep veins of left upper extremity | UMLS:ICD10CM:I82.722 | ||
| Chronic embolism and thrombosis of deep veins of upper extremity, bilateral | UMLS:ICD10CM:I82.723 | ||
| Lower Extremity DVT | Acute embolism and thrombosis of unspecified deep veins of unspecified lower extremity | UMLS:ICD10CM:I82.409 | |
| Chronic embolism and thrombosis of unspecified deep veins of unspecified lower extremity | UMLS:ICD10CM:I82.509 | ||
| Chronic embolism and thrombosis of unspecified deep veins of lower extremity | UMLS:ICD10CM:I82.50 | ||
| Chronic embolism and thrombosis of unspecified deep veins of lower extremity, bilateral | UMLS:ICD10CM:I82.503 | ||
| Acute embolism and thrombosis of unspecified deep veins of lower extremity | UMLS:ICD10CM:I82.40 | ||
| Acute embolism and thrombosis of unspecified deep veins of left lower extremity | UMLS:ICD10CM:I82.402 | ||
| Acute embolism and thrombosis of unspecified deep veins of right lower extremity | UMLS:ICD10CM:I82.401 | ||
| Chronic embolism and thrombosis of unspecified deep veins of left lower extremity | UMLS:ICD10CM:I82.502 | ||
| Chronic embolism and thrombosis of unspecified deep veins of right lower extremity | UMLS:ICD10CM:I82.501 | ||
| Chronic embolism and thrombosis of left femoral vein | UMLS:ICD10CM:I82.512 | ||
| Chronic embolism and thrombosis of right femoral vein | UMLS:ICD10CM:I82.511 | ||
| Acute embolism and thrombosis of right iliac vein | UMLS:ICD10CM:I82.421 | ||
| Chronic embolism and thrombosis of femoral vein, bilateral | UMLS:ICD10CM:I82.513 | ||
| Chronic embolism and thrombosis of unspecified deep veins of unspecified distal lower extremity | UMLS:ICD10CM:I82.5Z9 | ||
| Chronic embolism and thrombosis of unspecified tibial vein | UMLS:ICD10CM:I82.549 | ||
| Acute embolism and thrombosis of deep veins of lower extremity | UMLS:ICD10CM:I82.4 | ||
| Chronic embolism and thrombosis of deep veins of lower extremity | UMLS:ICD10CM:I82.5 | ||
| Chronic embolism and thrombosis of other specified deep vein of unspecified lower extremity | UMLS:ICD10CM:I82.599 | ||
| Acute embolism and thrombosis of unspecified deep veins of unspecified proximal lower extremity | UMLS:ICD10CM:I82.4Y9 | ||
| Superficial VT | Embolism and thrombosis of superficial veins of unspecified lower extremity | UMLS:ICD10CM:I82.819 | |
| Acute embolism and thrombosis of superficial veins of unspecified upper extremity | UMLS:ICD10CM:I82.619 | ||
| Chronic embolism and thrombosis of superficial veins of unspecified upper extremity | UMLS:ICD10CM:I82.719 | ||
| Bleeding | Hematemesis | UMLS:ICD10CM:K92.0 | |
| Hemoptysis | UMLS:ICD10CM:R04.2 | ||
| Hemorrhage from respiratory passages | UMLS:ICD10CM:R04 | ||
| Hemorrhage from other sites in respiratory passages | UMLS:ICD10CM:R04.8 | ||
| Hemorrhage from other sites in respiratory passages | UMLS:ICD10CM:R04.89 | ||
| Melena | UMLS:ICD10CM:K92.1 | ||
| Hemorrhage of anus and rectum | UMLS:ICD10CM:K62.5 | ||
| Epistaxis | UMLS:ICD10CM:R04.0 | ||
| Transfusion | Transfusion of Nonautologous Whole Blood into Peripheral Vein, Percutaneous Approach | UMLS:ICD10PCS:30233H1 | |
| Transfusion of Nonautologous Whole Blood into Central Vein, Percutaneous Approach | UMLS:ICD10PCS:30243H1 | ||
| Transfusion of Nonautologous Red Blood Cells into Peripheral Vein, Percutaneous Approach | UMLS:ICD10PCS:30233N1 | ||
| Transfusion, blood or blood components | UMLS:CPT:36430 | ||
| Transfusion of Nonautologous Red Blood Cells into Central Vein, Percutaneous Approach | UMLS:ICD10PCS:30243N1 | ||
| Transfusion of Nonautologous Frozen Red Cells into Peripheral Vein, Percutaneous Approach | UMLS:ICD10PCS:30233P1 | ||
| Transfusion of Nonautologous Red Blood Cells into Peripheral Artery, Percutaneous Approach (deprecated 2020) | UMLS:ICD10PCS:30253N1 | ||
| Transfusion of Nonautologous Frozen Red Cells into Central Vein, Percutaneous Approach | UMLS:ICD10PCS:30243P1 | ||
| Transfusion of Nonautologous Red Blood Cells into Central Artery, Percutaneous Approach (deprecated 2020) | UMLS:ICD10PCS:30263N1 | ||
| Transfusion of Nonautologous Frozen Red Cells into Peripheral Artery, Percutaneous Approach (deprecated 2020) | UMLS:ICD10PCS:30253P1 | ||
| Transfusion of Nonautologous Frozen Red Cells into Central Artery, Percutaneous Approach (deprecated 2020) | UMLS:ICD10PCS:30263P1 | ||
| Transfusion of blood product | UMLS:SNOMED:116859006 | ||
| Transfusion of red blood cells | UMLS:SNOMED:116863004 | ||
| Mortality | Deceased | Deceased (demographic) | |
Table 3: International Classification of Disease (ICD)-10 codes utilized to identify outcomes followed in the TriNetX database
DVT: Deep Venous Thrombosis; VT: Venous Thrombosis
Cohort definitions
For each medication listed, two cohorts were compared: (i) a cohort of patients with hypercortisolism on enoxaparin and (ii) a cohort of patients with hypercortisolism on heparin, warfarin, apixaban, or aspirin at 81 mg (Table 4). The cohorts strictly assessed only adult patients (defined as at least 18 years of age); pediatric patients were not analyzed.
| Cohort | Run |
| Enoxaparin | 146 HCOs with 99 providers responding with 12,885 patients |
| Heparin | 145 HCOs with 97 providers responding with 16,376 patients |
| Warfarin | 145 HCOs with 82 providers responding with 3,230 patients |
| Apixaban | 146 HCOs with 91 providers responding with 3,982 patients |
| Aspirin (81 mg) | 144 HCOs with 51 providers responding with 8,200 patients |
Table 4: Outputs of healthcare organization queries as defined in corresponding tables
HCO: Healthcare Organization
Statistical analysis
Index events and time windows were defined to analyze patient outcomes. The index event was defined as the first date a patient met the inclusion criteria for a cohort. The time window was defined as the five years after the index event during which a pre-defined outcome could occur. Outcomes of interest were identified using ICD-10 codes as outlined in Table 1, and included PE, UE DVT, LE DVT, superficial VT, bleeding, transfusion, and all-cause mortality. Cohorts were propensity score-matched 1:1 according to age at index event, sex, race and ethnicity, and comorbid conditions, including endocrine, cardiac, pulmonary, gastrointestinal, and genitourinary conditions (Table 5). Propensity score-matching was performed using TriNetX, with a greedy (nearest) neighbor matching algorithm (caliper of 0.1 pooled standard deviations).
| Variable | ICD-10 Code |
| Demographics | Age at Index (AI) |
| Female (F) | |
| Black/African American (2054-5) | |
| Male (M) | |
| White (2106-3) | |
| American Indian/Alaskan Native (1002-5) | |
| Unknown Race (UNK) | |
| Native Hawaiian/Other Pacific Islander (2076-8) | |
| Unknown Gender (UN) | |
| Not Hispanic/Latino (2186-5) | |
| Hispanic/Latino (2135-2) | |
| Other Race (2131-1) | |
| Asian (2028-9) | |
| Diagnosis | Endocrine, nutritional and metabolic diseases (E00-E89) |
| Factors influencing health status and contact with health services (Z00-Z99) | |
| Diseases of the musculoskeletal system and connective tissue (M00-M99) | |
| Diseases of the circulatory system (I00-I99) | |
| Diseases of the digestive system (K00-K95) | |
| Diseases of the nervous system (G00-G99) | |
| Diseases of the respiratory system (J00-J99) | |
| Diseases of the genitourinary system (N00-N99) | |
| Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism (D50-D89) | |
| Neoplasms (C00-D49) | |
| Diseases of the skin and subcutaneous tissue (L00-L99) |
Table 5: International Classification of Disease (ICD)-10 codes utilized to propensity match cohorts in the TriNetX database
Three analytical approaches were performed for this study, including measures of association, survival analysis, and frequency analysis. The measure of association analysis involved calculating RRs (and risk differences) with 95%CIs, comparing the proportion of patients across each cohort experiencing an outcome. Survival analysis was performed with Kaplan-Meier estimators (evaluating time-to-event outcomes), with Log-Rank testing incorporated to compare the survival curves. Furthermore, Cox proportional hazard models were incorporated to provide an estimate of the hazard ratios (HR) and 95%CIs. Patients who exited a cohort before the end of the time window were excluded from the survival analysis. The frequency analysis was performed by calculating the proportion of patients in each cohort who experienced an outcome during the defined period of five years.
For statistically significant associations, an E-value was calculated to assess the potential impact of unmeasured confounders, quantifying the minimum strength of association that would be required by an unmeasured confounder to explain the observed effect (beyond our measured covariates); an E-value of above 2.0 was considered modestly robust, and above 3 was considered strongly robust. Additionally, a limited sensitivity analysis assessing Pituitary Cushing’s (the most common cause of endogenous Cushing’s Syndrome) was performed. All analyses were conducted through TriNetX, with statistical significance defined as a p-value < 0.05.
Results
Cushing’s syndrome, unspecified
Enoxaparin and Heparin
After propensity-score matching, 8,658 patients were identified in each cohort. The average age at index event for the enoxaparin cohort was 54.5 + 16.5 years, compared to 53.1 + 17.3 years for the heparin cohort. The enoxaparin cohort had 6,216 females (71.8%), compared to 6,000 (69.3%) in the heparin cohort. Within the enoxaparin cohort, 6035 (69.7%) were Caucasian patients, followed by 987 (11.4%) African American patients, 753 (8.7%) Hispanic/Latino patients, and 216 (2.5%) Asian patients. The heparin cohort was similar in ethnicity, with 5,800 (67.0%) Caucasian patients, 1,099 (12.7%) African American patients, 753 (8.7%) Hispanic/Latino patients, and 268 (3.1%) Asian patients. The enoxaparin and heparin cohorts demonstrated no significant differences in PE (HR 1.171, 95%CI 1.017-1.348, p=0.1797), UE DVT (HR 1.067, 95%CI 0.837-1.362, p=0.8051), LE DVT (HR 1.066, 95%CI 0.931-1.222, p=0.1922), superficial VT (HR 0.974, 95%CI 0.672-1.41, p=0.4576), bleeding (HR 0.948, 95%CI 0.855-1.05, p=0.3547), transfusion (HR 0.873, 95%CI 0.786-0.969, p=0.1767), or all-cause mortality (HR 1.036, 95%CI 0.966-1.11, p=0.9954). A comprehensive summary of the results is demonstrated in Table 6.
| p-value | Medication 1 | Medication 2 | PE | UE DVT | LE DVT | S VT | Bleeding | Transfusion | Mortality |
| enoxaparin | heparin | 0.1797 | 0.8051 | 0.1922 | 0.4576 | 0.3547 | 0.1767 | 0.9954 | |
| enoxaparin | warfarin | 0.3828 | 0.6 | 0.1963 | 0.0995 | 0.7768 | 0.5715 | 0.15 | |
| enoxaparin | apixaban | 0.6491 | 0.6275 | 0.723 | 0.4198 | 0.4356 | 0.4299 | 0.2628 | |
| enoxaparin | aspirin 81 mg | 0.0345 | 0.587 | 0.0017 | 0.4218 | 0.246 | 0.2057 | 0.0002 | |
| – | – | – | – | – | – | – | – | – | – |
| HR | Medication 1 | Medication 2 | PE | UE DVT | LE DVT | S VT | Bleeding | Transfusion | Mortality |
| enoxaparin | heparin | 1.171 | 1.067 | 1.066 | 0.974 | 0.948 | 0.873 | 1.036 | |
| enoxaparin | warfarin | 0.936 | 0.969 | 0.708 | 0.655 | 0.961 | 1.127 | 1.042 | |
| enoxaparin | apixaban | 0.798 | 0.666 | 0.684 | 4.059 | 0.933 | 1.089 | 1.041 | |
| enoxaparin | aspirin 81 mg | 1.697 | 1.398 | 1.492 | 1.718 | 1.107 | 1.347 | 1.272 | |
| – | – | – | – | – | – | – | – | – | – |
| 95% CIs | Medication 1 | Medication 2 | PE | UE DVT | LE DVT | Superficial VT | Bleeding | Transfusion | Mortality |
| enoxaparin | heparin | 1.017-1.348 | 0.837-1.362 | 0.931-1.222 | 0.672-1.41 | 0.855-1.05 | 0.786-0.969 | 0.966-1.11 | |
| enoxaparin | warfarin | 0.755-1.161 | 0.692-1.356 | 0.583-0.859 | 0.376-1.142 | 0.812-1.137 | 0.95-1.336 | 0.93-1.167 | |
| enoxaparin | apixaban | 0.608-1.047 | 0.431-1.03 | 0.593-0.788 | 1.156-14.258 | 0.771-1.129 | 0.892-1.33 | 0.912-1.189 | |
| enoxaparin | aspirin 81 mg | 1.444-1.994 | 1.06-1.845 | 1.28-1.738 | 1.011-2.92 | 0.986-1.243 | 1.185-1.532 | 1.167-1.386 | |
Table 6: Hazard Ratio, 95% Confidence Intervals and p-values for anticoagulation and antiplatelet comparisons in all causes of Cushing’s Syndrome
HR: hazard ratio; CI: confidence interval; PE: pulmonary embolism; VT: venous thrombosis; DVT: deep vein thrombosis; UE: upper extremity; LE: lower extremity
Enoxaparin and Warfarin
After propensity-score matching, 2,786 patients were identified in each cohort. The average age at index event for the enoxaparin cohort was 54.8 + 16.4 years, compared to 58.9 + 15.9 years for the warfarin cohort. The enoxaparin cohort had 2,020 female patients (72.5%) compared to 1,861 (66.8%) in the warfarin cohort. Within the enoxaparin cohort, 2,000 (71.8%) were Caucasian patients, followed by 334 (12.0%) African American patients, 220 (7.98%) Hispanic/Latino patients, and 64 (2.3%) Asian patients. The warfarin cohort was similar, with 2,056 (73.8%) Caucasian patients, 312 (11.2%) African American patients, 170 (6.1%) Hispanic/Latino patients, and 92 (3.3%) Asian patients. The enoxaparin and warfarin cohorts demonstrated no significant differences in PE (HR 0.936, 95%CI 0.755-1.161, p=0.3828), UE DVT (HR 0.969, 95%CI 0.692-1.356, p=0.6), LE DVT (HR 0.708, 95%CI 0.583-0.859, p=0.1963), superficial VT (HR 0.655, 95%CI 0.376-1.142, p=0.0995), bleeding (HR 0.961, 95%CI 0.812-1.137, p=0.7768), transfusion (HR 1.127, 95%CI 0.95-1.336, p=0.5715), or all-cause mortality (HR 1.042, 95%CI 0.93-1.167, p=0.15) (Table 6).
Enoxaparin and Apixaban
After propensity-score matching, 2,429 patients were identified in each cohort. The average age at index event for the enoxaparin cohort was 54.6 + 16.4 years, compared to 61.2 + 15.2 years for the apixaban cohort. The enoxaparin cohort had 1,746 female patients (71.9%) compared to 1,571 (64.7%) in the apixaban cohort. Within the enoxaparin cohort, 1632 (67.2%) were Caucasian patients, 318 (13.1%) African American patients, 219 (9.0%) Hispanic/Latino patients, and 68 (2.8%) Asian patients. A similar composition was noted in the apixaban cohort, with 1,683 (69.3%) Caucasian patients, 321 (13.2%) African American patients, 141 (5.8%) Hispanic/Latino patients, and 53 (2.2%) Asian patients. The enoxaparin and apixaban cohorts demonstrated no significant differences in PE (HR 0.798, 95%CI 0.608-1.047, p=0.6491), UE DVT (HR 0.666, 95%CI 0.431-1.03, p=0.6275), LE DVT (HR 0.684, 95%CI 0.593-0.788, p=0.723), superficial VT (HR 4.059, 95%CI 1.156-14.258, p=0.4198), bleeding (HR 0.933, 95%CI 0.771-1.129, p=0.4356), transfusion (HR 1.089, 95%CI 0.892-1.33, p=0.4299), or all-cause mortality (HR 1.041, 95%CI 0.912-1.189, p=0.2628) (Table 6).
Enoxaparin and Aspirin 81 mg
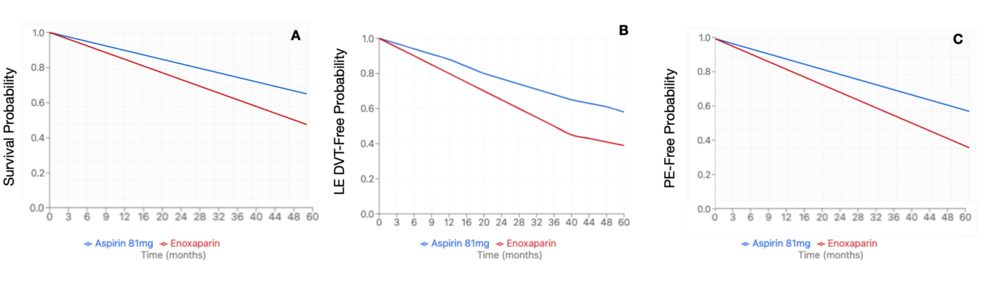
After propensity-score matching, 6,433 patients were identified in each cohort. The average age at index event for the enoxaparin cohort was 54.5 + 16.6 years, compared to the aspirin 81 mg cohort at 58.8 + 14.9 years. The enoxaparin cohort had 4664 female patients (72.5%) compared to 4,445 (69.1%) in the aspirin 81 mg cohort. Within the enoxaparin cohort, 4,522 (70.3%) were Caucasian patients, followed by 766 (11.9%) African American patients, 521 (8.1%) Hispanic/Latino patients, and 193 (3.0%) Asian patients. Similar demographics were noted within the Aspirin 81 mg cohort, with 4,670 (72.6%) Caucasian patients, 817 (12.7%) African American patients, 425 (6.6%) Hispanic/Latino patients, and 167 (2.6%) Asian patients. The enoxaparin cohort demonstrated a significantly higher risk of PE (HR 1.697, 95%CI 1.444-1.994, p=0.0345), LE DVT (HR 1.492, 95%CI 1.28-1.738, p=0.0017), and all-cause mortality (HR 1.272, 95%CI 1.167-1.386, p=0.0002) compared to the aspirin 81 mg cohort (Figure 2). There was no significant difference in rates of UE DVT (HR 1.398, 95%CI 1.06-1.845, p=0.587), superficial VT (HR 1.718, 95%CI 1.011-2.92, p=0.4268), bleeding (HR 1.107, 95%CI 0.986-1.243, p=0.246), or transfusion (HR 1.347, 95%CI 1.185-1.532, p=0.2057) (Table 6). Due to a significant difference between enoxaparin and Aspirin 81 mg, an E-value was calculated for PE (E-value = 2.783), LE DVT (E-value = 2.348), and all-cause mortality (E-value = 1.860).
Figure 2: Kaplan-Meier survival curve for pituitary Cushing’s subtype (mortality, LE DVT, and PE)
(A) Mortality of enoxaparin compared to aspirin 81mg (HR 1.272, 95% CI 1.167-1.386, p=0.0002); (B) LE DVT risk with enoxaparin compared to aspirin 81 mg (HR 1.492, 95%CI 1.28-1.738, p=0.0017); (C) PE risk with enoxaparin compared to aspirin 81 mg (HR: 1.697, 95%CI 1.444-1.994, p=0.0345)
DVT: deep vein thrombosis; LE: lower extremity; PE: pulmonary embolism
Pituitary hypercortisolism (Cushing’s disease)
Enoxaparin and Heparin
Propensity-score matching identified 5,602 patients per cohort. The average age at index for the enoxaparin cohort was 53.9 + 16.7 years, compared to 53.7 + 16.9 years in the heparin cohort. The enoxaparin cohort had 4,088 female patients (72.97%) compared to 4,066 (72.58%) in the heparin cohort. The enoxaparin cohort was predominantly Caucasian patients (n=3,948; 70.47%), followed by 641 (11.45%) African American patients, 424 (7.57%) Hispanic/Latino patients, and 139 (2.48%) Asian patients. The heparin cohort was also predominantly Caucasian (n=3,947; 70.46%), followed by 669 (11.94%) African American patients, 401 (7.16%) Hispanic/Latino patients, and 148 (2.64%) Asian patients. There were no significant differences in rates of PE (HR 1.208, 95%CI 1.007 – 1.451, p=0.5803), UE DVT (HR 1.156, 95%CI 0.841 – 1.59, p=0.6863), LE DVT (HR 1.246, 95%CI 1.063 – 1.46, p=0.8996), superficial VT (HR 1.347, 95%CI 0.874 – 2.075, p=0.3731), bleeding (HR 0.916, 95%CI 0.809 – 1.037, p=0.1578), transfusion (HR 0.912, 95%CI 0.798 – 1.042, p=2119), or all-cause mortality (HR 1.02, 95%CI 0.935 – 1.112, p=0.8734). A comprehensive summary of the results is demonstrated in Table 7.
| p-value | Medication 1 | Medication 2 | PE | UE DVT | LE DVT | Superficial VT | Bleeding | Transfusion | Mortality |
| enoxaparin | heparin | 0.5189 | 0.2468 | 0.7586 | 0.7708 | 0.5894 | 0.6273 | 0.8433 | |
| enoxaparin | warfarin | 0.4842 | 0.7763 | 0.9651 | 0.682 | 0.1996 | 0.5309 | 0.399 | |
| enoxaparin | apixaban | 0.1047 | 0.0423 | 0.647 | 0.4824 | 0.2698 | 0.1122 | 0.1044 | |
| enoxaparin | aspirin 81 mg | 0.9651 | 0.6358 | 0.8448 | 0.9765 | 0.1167 | 0.4854 | 0.5001 | |
| – | – | – | – | – | – | – | – | – | – |
| HR | Medication 1 | Medication 2 | PE | UE DVT | LE DVT | Superficial VT | Bleeding | Transfusion | Mortality |
| enoxaparin | heparin | 1.186 | 1.332 | 1.232 | 1.183 | 0.876 | 0.963 | 1.016 | |
| enoxaparin | warfarin | 0.804 | 0.76 | 0.688 | 0.815 | 1.008 | 1.009 | 0.976 | |
| enoxaparin | apixaban | 0.875 | 0.761 | 0.954 | 3.068 | 1.084 | 1.359 | 1.115 | |
| enoxaparin | aspirin 81 mg | 1.173 | 1.157 | 1.226 | 1.165 | 0.908 | 0.915 | 1.028 | |
| – | – | – | – | – | – | – | – | – | – |
| 95% CIs | Medication 1 | Medication 2 | PE | UE DVT | LE DVT | Superficial VT | Bleeding | Transfusion | Mortality |
| enoxaparin | heparin | 0.983-1.433 | 0.941-1.885 | 1.032-1.47 | 0.776-1.803 | 0.769-0.998 | 0.808-1.147 | 0.929-1.112 | |
| enoxaparin | warfarin | 0.612-1.055 | 0.467-1.235 | 0.539-0.877 | 0.447-1.489 | 0.816-1.246 | 0.76-1.34 | 0.843-1.13 | |
| enoxaparin | apixaban | 0.659-1.162 | 0.456-1.271 | 0.736-1.236 | 0.843-11.166 | 0.845-1.381 | 0.962-1.921 | 0.944-1.317 | |
| enoxaparin | aspirin 81mg | 0.969-1.419 | 0.827-1.619 | 1.03-1.46 | 0.763-1.78 | 0.797-1.035 | 0.772-1.085 | 0.938-1.127 | |
Table 7: Hazard ratio, 95% confidence intervals, and p-values for anticoagulation and antiplatelet comparisons in pituitary Cushing’s syndrome
HR: hazard ratio; CI: confidence interval; PE: pulmonary embolism; VT: venous thrombosis; DVT: deep vein thrombosis; UE: upper extremity; LE: lower extremity
Enoxaparin and Warfarin
Propensity-score matching was performed with 1,694 patients per cohort identified. The average age at index for the enoxaparin cohort was 58.1 + 15.8 years, compared to 58.1 + 15.9 years in the warfarin cohort. The enoxaparin cohort had 1,142 female patients (67.41%) compared to 1,143 (67.47%) in the warfarin cohort. Within the enoxaparin cohort, 1,224 (72.2%) were Caucasian patients, followed by 194 (11.45%) African American patients, 97 (5.73%) Hispanic/Latino patients, and 57 (3.37%) Asian patients. The warfarin cohort had similar demographics, with 1,223 (72.2%) Caucasian patients, followed by 194 (11.45%) African American patients, 102 (6.02%) Hispanic/Latino patients, and 65 (3.84%) Asian patients. There were no significant differences in rates of PE (HR 0.907, 95%CI 0.694 – 1.186, p=0.8117), UE DVT (HR 0.988, 95%CI 0.628 – 1.555, p=0.9848), LE DVT (HR 0.739, 95%CI 0.589 – 0.929, p=0.4445), superficial VT (HR 0.815, 95%CI 0.44 – 1.511, p=0.8098), bleeding (HR 1.001, 95%CI 0.814 – 1.231, p=0.0987), transfusion (HR 1.106, 95%CI 0.889 – 1.376, p=0.4904), or all-cause mortality (HR 0.951, 95%CI 0.83 – 1.089, p=0.1656) (Table 7).
Enoxaparin and Apixaban
Propensity-score matching identified 1,489 patients per cohort. The enoxaparin cohort was 61.1 + 15.1 years old at the index event, versus the apixaban cohort at 61.4 + 14.9 years. The enoxaparin cohort had 1,054 (70.79%) female patients compared with 1,029 (69.11%) in the apixaban cohort. The enoxaparin cohort was primarily Caucasian patients (n=1,105; 74.21%), followed by 179 (12.02%) African American patients, 74 (4.97%) Hispanic/Latino patients, and 27 (1.81%) Asian patients. The apixaban cohort demonstrated similar demographics with 1,080 (72.53%) Caucasian patients, followed by 180 (12.09%) African American patients, 76 (5.1%) Hispanic/Latino patients, and 27 (1.81%) Asian patients. There were no significant differences in rates of PE (HR 0.949, 95%CI 0.673 – 1.339, p=0.4372), UE DVT (HR 0.832, 95%CI 0.472 – 1.466, p=0.1538), LE DVT (HR 1.166, 95%CI 0.869 – 1.566, p=0.8595), superficial VT (HR 5.323, 95%CI 1.19 – 23.815, p=0.493), bleeding (HR 1.218, 95%CI 0.948 – 1.565, p=0.4021), transfusion (HR 1.319, 95%CI 0.993 – 1.753, p=0.1663), or all-cause mortality (HR 1.131, 95%CI 0.966 – 1.325, p=0.0839) (Table 7).
Enoxaparin and Aspirin 81 mg
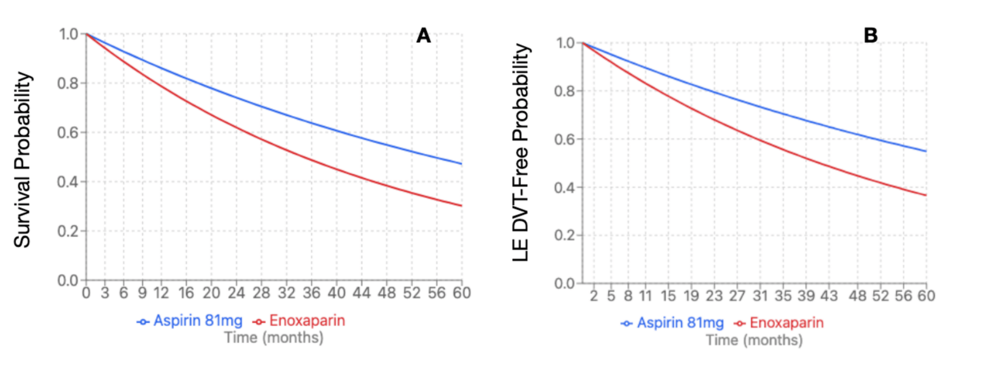
Propensity-score matching revealed 3,475 patients per cohort. The enoxaparin cohort was 58.8 + 15.3 years at index event, compared to the aspirin cohort at 58.2 + 14.3 years. The enoxaparin cohort had 2,438 (70.16%) female patients compared to the aspirin cohort with 2,445 (70.36%). Within the enoxaparin cohort, 2,539 (73.06%) were Caucasian patients, followed by 378 (10.88%) African American patients, 182 (5.24%) Hispanic/Latino patients, and 74 (2.13%) Asian patients. The aspirin cohort demonstrated similar demographics with 2,554 (73.5%) Caucasian patients, followed by 363 (10.45%) African American patients, 196 (5.64%) Hispanic/Latino patients, and 68 (1.96%) Asian patients. The enoxaparin cohort demonstrated significantly increased risk of LE DVT (HR 1.677, 95%CI 1.353 – 2.079, p=0.0081) and all-cause mortality (HR 1.597, 95%CI 1.422 – 1.794, p=0.0005) (Figure 3). There were no significant differences in rates of PE (HR 1.74, 95%CI 1.354 – 2.236, p=0.2408), UE DVT (HR 1.773, 95%CI 1.108 – 2.837, p=0.8625), superficial VT (HR 4.273, 95%CI 1.969 – 9.273, p=0.5196), bleeding (HR 1.093, 95%CI 0.937 – 1.275, p=0.8554), or transfusion (HR 1.896, 95%CI 1.556 – 2.311, p=0.2609) (Table 7). Due to a significant difference between enoxaparin and Aspirin 81 mg, an E-value was calculated for LE DVT (E-value = 2.744) and all-cause mortality (E-value = 2.574).
Figure 3: Kaplan-Meier survival curve for pituitary Cushing’s subtype (mortality and LE DVT)
(A) Mortality of enoxaparin compared to aspirin 81 mg (HR 1.597, 95%CI 1.422-1.794, p=0.0005); (B) LE DVT of enoxaparin compared to aspirin 81 mg (HR 1.677, 95%CI: 1.353-2.079, p=0.0081)
HR: hazard ration; DVT: deep vein thrombosis; LE: lower extremity
Discussion
The concept of hypercoagulability in the setting of hypercortisolemia has been documented since the 1970s [10]. Estimates suggest an 18-fold risk of venous thromboembolism in patients with Cushing’s syndrome compared to the general population [11]. Furthermore, venous thromboembolism accounts for up to 11% of all deaths in Cushing’s syndrome [12]. Patients are often noted to have a “coagulation paradox” in Cushing’s syndrome, whereby there is a heightened risk for thrombosis, with concurrent bruising of the skin; thromboembolism is due to an imbalance between pro- and anti-coagulant pathways, whereas bruising is due to atrophy of the skin and capillary fragility [11]. As noted by Feelders and Nieman, two prominent phases for the development of thromboembolic events include the untreated (active) hypercortisolemia and the postoperative phases [11]. Population-based studies have demonstrated a heightened risk for venous thromboembolism prior to diagnosis (in some studies as early as three years before diagnosis) [9].
Despite this heightened risk for venous thromboembolic events, there appears to be a lack of awareness amongst institutions (and individual practitioners), along with improper management. Fleseriu and colleagues, however, do note that in 2020, the awareness of hypercoagulability in Cushing’s syndrome increased around fourfold in two years, with routine prophylaxis increasing to 75% (from 50%) perioperatively (however, most patients only received prophylaxis for up to two weeks postoperatively) [13]. Another survey was performed by the European Reference Network on Rare Endocrine Conditions, noting concerns of heterogeneity with timing, type, and duration of prophylaxis, noting most centers do not have a thromboprophylaxis protocol (identifying only one reference center had a standardized thromboprophylaxis protocol for Cushing’s syndrome) [14]. From the European survey, it was noted that prophylaxis was initiated at diagnosis in 48% of patients, with 17% preoperatively, 26% on the day before (or of) surgery, 13% postoperatively, and 9% “depending on the presentation”. With regards to discontinuation of thromboprophylaxis, in centers with a standardized protocol (35% of reference centers), 38% of centers stopped at one month post-operatively, 25% between two and four weeks, and 37% between one week before and two weeks after surgery, between four and six days postoperatively, and at three months postoperatively. When cessation was individualized (in the remaining 65% of reference centers), 60% discontinued thromboprophylaxis once the patient was mobile, 40% with achievement of remission, 27% regarding patient status, and 7% dependent upon hemostatic parameters [14].
There is limited guidance concerning thromboprophylaxis recommendations in Cushing’s syndrome. For example, the Endocrine Society merely recommends assessing the risk of thrombosis in Cushing’s syndrome and administering perioperative prophylaxis if undergoing surgery, but provides no further recommendations [8]. The Pituitary Society highlights the absence of standardized practice for both pre- and postoperative thromboprophylaxis in patients with Cushing’s syndrome [15]. There appears to only be one set of guidelines for thromboprophylaxis in Cushing’s syndrome, known as the “Delphi Panel Consensus”, which forms the basis for the guidelines from the European Society for Endocrinology [9]. The Delphi Panel Consensus recommends considering anticoagulation for all patients with Cushing’s syndrome (in the absence of contraindications), regardless of the underlying etiology, and is recommended in the presence of risk factors [9]. Moreover, thromboprophylaxis is advised to begin at the time of diagnosis [9]. Currently, there is not enough evidence to provide a recommendation for thromboprophylaxis in mild autonomous cortisol secretion [9]. As with any medical patient, thromboprophylaxis should be initiated in all patients with active Cushing’s syndrome who are hospitalized (without contraindications) [9, 15]. Apart from chemical prophylaxis, anti-embolic stockings are not recommended due to the risk of skin fragility and friability [9]. The Delphi Consensus Panel furthermore advises to continue prophylactic anticoagulation for at least three months after biochemical remission (eucortisolemia) has occurred, and note those without additional risk factors (such as obesity, immobility, prior history of venous thromboembolism, or cardiac risk factors) can be considered candidates to stop the medication; one caveat, however, is for patients medically managed with mitotane (which can alter liver function and coagulation factor metabolism), there is an increased risk of bleeding, for which careful monitoring of renal function and bleeding risk is advised [9]. The Pituitary Society provides additional recommendations, such as discontinuing estrogen therapy in women (if used for contraception) [15]. While the Delphi Consensus Panel does not comment upon pediatric patients, the Pituitary Society advises against the use of thromboprophylaxis in the pediatric population due to bleeding risks [15].
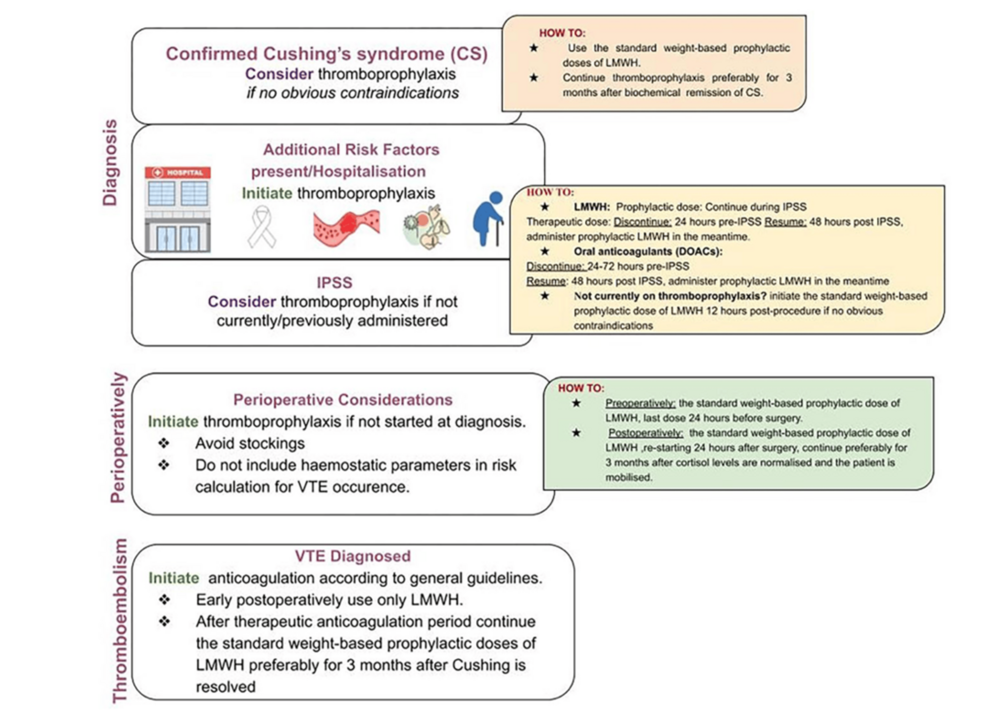
The Delphi Consensus Panel furthermore recommend considering thromboprophylaxis at the time of inferior petrosal sinus sampling (if not started before this), due to the risk of thrombosis associated with this intervention; for those who are receiving prophylaxis, it is recommended to continue throughout the procedure, however, if has not been started, it is advised to initiate 12 hours post procedure. Similarly, if thromboprophylaxis was not considered earlier in a patient’s course, it should be reconsidered in the perioperative period, with the last dose of LMWH administered 24 hours prior to surgery and reinitiated 24 hours postoperatively [9]. Isand et al. recommend continuing thromboprophylaxis for three months after cortisol levels normalize (< 5 μg/dL) and when patients can mobilize [9]. In patients for whom a venous thromboembolism develops, patients are advised to receive a therapeutic dose of anticoagulation (preferably LMWH) for three to six months, followed by prophylaxis for three months after resolution of Cushing’s syndrome [9]. The Delphi Consensus Panel provides a summary of their recommendations, shown in Figure 4.
Figure 4: Algorithm for thromboprophylaxis in Cushing’s syndrome
IPSS: inferior petrosal sinus sampling; VTE: venous thromboembolism; LMWH: low-molecular-weight heparin; DOAC: direct oral anticoagulant
Source: Isand et al., 2025 [9]; Published with permission.
Although intuitively, one may expect the procoagulant profile of Cushing’s syndrome to resolve upon attainment of eucortisolemia with medical management, studies have failed to demonstrate a reduction in venous thromboembolism with medical therapy [16]. Additionally, while one may expect resolution of hypercoagulability with surgical intervention (transsphenoidal sinus surgery or adrenalectomy), the risk maintains in the postoperative period, comparable to that of orthopedic surgery, at times up to one year and beyond to normalize [17]; data from European Register on Cushing’s Syndrome (ERCUSYN) database suggest the risk is greatest six months postoperatively [18]. The estimated risk for postoperative venous thromboembolism in pituitary-dependent Cushing’s is around 4.3% (compared to 0% with a non-functional pituitary adenoma); regarding adrenal surgery, the risk is estimated at around 2.6% [11]. Although the underlying mechanism for the persistent risk for venous thromboembolism remains unknown, it is hypothesized that a sudden drop in cortisol can lead to an inflammatory response (itself activating the coagulation cascade) [16]. Lopes and colleagues note an increase in the number of lymphocytes (because of loss of Th1 cell suppression), with increases in cytokines (such as interferon-gamma, interleukin-2, and transforming growth factor-beta) [16]. Comorbidities such as osteoporosis and myopathy (from hypercortisolemia) may be associated with decreased mobility in the postoperative period, influencing the risk for thrombosis [16].
Whilst all subtypes of Cushing’s syndrome can be associated with a heightened risk for venous thromboembolism (pituitary adenoma, adrenal adenoma, medication-induced, ectopic ACTH, and adrenal carcinoma), the latter two are often associated with malignant disease, which itself poses a risk for hypercoagulability from the underlying neoplasm [11]. Patients with Cushing’s syndrome have been found to demonstrate a reduction in activated partial thromboplastin time (aPTT), alongside increases in clot lysis time, procoagulant factors (such as factor VIII, von-Willebrand factor and fibrinogen) and fibrinolysis inhibitors (including plasminogen activator-inhibitor-1, thrombin activatable fibrinolysis inhibitor, and alpha-2 antiplasmin) [11,12,17]. Varlamov et al. have also noted an increase in thrombin, thromboxane A2, and platelets. Other studies have additionally demonstrated elevated proteins C and S as well as antithrombin III, which are hypothesized to be increased as a compensatory mechanism from the state of hypercoagulability [12]. Barbot et al. demonstrate elevation in factor VIII and von-Willebrand factor within the first few months after transsphenoidal sinus surgery, along with abnormally large von-Willebrand multimers (which are typically found in the cellular components), which can induce spontaneous platelet aggregation [17].
Lopes et al. note that altered von-Willebrand factor levels are not a constant feature reported in Cushing’s syndrome, and state it depends upon the polymorphism of the gene promoter, providing an example of haplotype 1 of the gene promoter conferring the greatest risk for elevated von-Willebrand factor levels by cortisol [16]. Barbot and colleagues furthermore note ABO blood groupings as an additional influencer of the procoagulant state; as an example, blood group-O patients have a near one-quarter reduction in levels of von-Willebrand factor [17]. Feelders and Nieman note heterogeneity in coagulation profiles based on individual characteristics and differing assay techniques [11]. van Haalen and colleagues note an absence of a correlation between severity of hypercortisolism and hemostatic abnormalities [14]; this is echoed by Varlamov et al., stating there is no linear relationship between coagulation parameters and venous thromboembolic events, nor with urinary free cortisol elevation [12]. Varlamov and colleagues further note that a subset of patients may have unaltered coagulation parameters, for which they advise against stratifying patients’ risk based on coagulation parameters [12].
In 2016, Zilio and colleagues posed a scoring system to stratify patients with active Cushing’s syndrome, including both clinical and biochemical parameters, including age (> 69 = 2 points), reduction in mobility (2 points), acute severe infection (1 point), prior cardiovascular event(s) (1 point), midnight plasma cortisol (> 3.15 times upper limit of normal = 1 point), and shortened aPTT (1 point) [19]. Lopes et al. describe the stratification as follows: 2 points (low risk), 3 points (moderate risk), 4 points (high risk), and > 5 points (very high risk) [16]. It should be noted, however, that Zilio et al.’s study was performed on only 176 patients and has not been validated in other studies [19]. Further drawbacks include the failure to account for postoperative events (a major source of venous thromboembolism in Cushing’s syndrome), and despite the stratification categories, no recommendations for treatment are provided.
LMWH is the first-line medication, consistent across differing societies. Despite being the gold standard, there are limited studies demonstrating a beneficial reduction in venous thromboembolic events in such cohorts; similarly, studies are lacking in analysis of the other classes of anticoagulants in head-to-head comparisons against LMWH for thromboprophylaxis in hypercortisolism. Another limitation is the fact that certain studies solely address thromboprophylaxis in the postoperative period. As an example, McCormick et al. performed one of the only trials comparing unfractionated heparin and LMWH (enoxaparin), noting no differences in hemorrhagic complications or thromboses; however, this was analyzed in patients undergoing transsphenoidal sinus surgery [10].
The current study retrospectively analyzed the various anticoagulant agents for the prevention of venous thromboembolism in Cushing’s syndrome (of any subtype), compared to the gold standard, LMWH (in this study, enoxaparin). When analyzing Cushing’s syndrome, our study demonstrated no significant differences in outcomes between enoxaparin and warfarin, apixaban, or unfractionated heparin; however, aspirin 81 mg demonstrated a lower risk of all-cause mortality, PE, and LE DVT. With subanalysis of Cushing’s disease (pituitary-related), there was no significant difference between enoxaparin and warfarin, apixaban or unfractionated heparin; aspirin 81 mg again noted a reduced all-cause mortality and LE DVT (but did not lower the risk of PE, compared with Cushing’s syndrome of all types combined). With E-value sensitivity analysis, the association remained moderately robust with PE (all Cushing’s types combined), LE DVT (all Cushing’s types and pituitary Cushing’s), and mortality (solely pituitary Cushing’s), however, mortality was weak-to-moderate with Cushing’s syndrome of all types (Table 8).
| Outcome | Hazard Ratio | E-value | Interpretation |
| PE (All Cushing’s Types) | 1.697 | 2.783 | Moderate |
| LE DVT (All Cushing’s Types) | 1.492 | 2.348 | Moderate |
| LE DVT (Pituitary) | 1.677 | 2.744 | Moderate |
| Mortality (All Cushing’s Types) | 1.272 | 1.860 | Weak |
| Mortality (Pituitary) | 1.597 | 2.574 | Moderate |
Table 8: E-value sensitivity analyses for significant findings
DVT: deep vein thrombosis; LE: lower extremity; PE: pulmonary embolism
Aspirin, a non-steroidal anti-inflammatory drug, was first identified to irreversibly inhibit platelet function in the 1950s by Dr. Lawrence Craven [20]. Data is scarce in terms of aspirin’s role in thromboprophylaxis in hypercortisolemia. In 1999, Semple and Laws Jr. initially reported the use of aspirin postoperatively for six weeks (starting postoperative day one) in patients with Cushing’s disease who underwent transsphenoidal sinus surgery; while the authors mentioned a reduction in rates of venous thromboemboli, no factual data was provided (including dose of aspirin, complications experienced, and number of venous thromboemboli before and after) [21]. In 2015, Smith et al. performed an additional study with 81 mg of aspirin again administered starting postoperative day one (alongside sequential compression devices and mobilization), reporting that none of the 82 patients developed DVTs (with only two cases of epistaxis) [22]. It was not until 1994, however, in the Antiplatelet Trialists’ Collaborations’ meta-analysis, that aspirin demonstrated a reduced risk for venous thromboembolism, with similar findings replicated in the Pulmonary Embolism Prevention trial in 2000 and the WARFASA (Warfarin and Aspirin) and ASPIRE (Aspirin to prevent recurrent venous thromboembolism) trials in 2012 [23]. In 2012, the American College of Chest Physicians [24,25] were the first to recommend aspirin as thromboprophylaxis following total hip or knee replacement, followed by the National Institute for Health and Care Excellence in 2018 (advising LMWP followed by aspirin) and the American Society of Hematology in 2019 (advising either aspirin or oral anticoagulation after total hip or knee replacement) [25]. Despite recognition of the reduction in venous thromboembolism by aspirin (and its incorporation into guidelines), its role in thromboprophylaxis is largely limited to orthopedic surgery. The mechanisms of aspirin and its reduction in venous thromboembolism is not entirely understood, but believed to occur via differing mechanisms, including inhibition of cyclooxygenase-1 (which reduces thromboxane A2, a promoter of platelet aggregation), prevention of thrombin formation and thrombin-mediated coagulant reactions, acetylation of proteins involved in coagulation (such as fibrinogen), and enhancing fibrinolysis [23,26].
Strengths and limitations
To the best of our knowledge, a study specifically comparing the impact of aspirin with that of LMWP in Cushing’s syndrome has not been performed; as a result, our study adds to the paucity of literature pertaining to this topic. Notable strengths in the study include a large sample size (allowing robust comparisons amongst treatment arms), incorporation of propensity-score matching (allowing for internal validity through balancing baseline comparison groups), and comprehensive measurable outcomes.
Limitations to our study are multifold, and include retrospective design, for which intrinsic biases are inherent and can affect causal inference (despite matching techniques). Furthermore, data collection (via TriNetX) relied on correct ICD-10 coding, which could be a source of potential error if conditions and medications are coded improperly, or if our queries missed ICD-10 codes that could also correspond with outcomes. Similarly, TriNetX also relies on queries of healthcare organizations, many of which may not have responded with data, which could inaccurately skew the results. Although TriNetX uses global data, the majority of patient data was derived from the United States population, which could result in less generalizable data to the global public. These findings should be interpreted within the correct context and with caution to prevent misrepresentation. Compliance was a variable that could not be controlled for. Moreover, those who had taken the medication before the index event were excluded from analysis. While aspirin 81 mg demonstrated a reduction in LE DVT and mortality in Cushing’s disease along with PE with Cushing’s syndrome, we only performed a subgroup analysis concerning pituitary-related causes of Cushing’s syndrome (Cushing’s disease); it remains unclear why the risk of PE was not reduced in the latter subgroup. Due to limitations in ICD-10 coding, further subgroup analyses were not performed (such as adrenal adenoma, adrenal adenocarcinoma, or ectopic ACTH syndrome), for which the implications of treating with aspirin 81 mg cannot be inferred from our data. Similarly, further subgroup analyses, such as gender and race, were not performed. Our study assessed adult patients with Cushing’s syndrome, and not pediatric patients, which limits the applicability of our findings to such a cohort. Further studies are required to confirm and replicate our findings in a prospective fashion, stratifying subtypes of Cushing’s Syndrome.
Conclusions
Cushing’s syndrome is associated with a heightened risk for venous thromboembolism, regardless of the underlying etiology. Currently, LMWHs such as enoxaparin remain the gold standard for both thromboprophylaxis and treatment in such patients. There is limited data to support superiority over alternative agents. Our study analyzed enoxaparin against warfarin, unfractionated heparin, and apixaban, for which there was no significant risk difference. When compared to aspirin, enoxaparin demonstrated a greater risk for the development of PE, LE DVT, and all-cause mortality. Further prospective trials are required to replicate our findings and confirm the superiority of aspirin over LMWH.
References
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP: Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006, 291:E965-73. 10.1152/ajpendo.00070.2006
- Lindholm J: Cushing’s syndrome: historical aspects. Pituitary. 2000, 3:97-104. 10.1023/a:1009905808033
- Raff H, Carroll T: Cushing’s syndrome: from physiological principles to diagnosis and clinical care. J Physiol. 2015, 593:493-506. 10.1113/jphysiol.2014.282871
- Newell-Price J, Bertagna X, Grossman AB, Nieman LK: Cushing’s syndrome. Lancet. 2006, 367:1605-17. 10.1016/S0140-6736(06)68699-6
- Savas M, Mehta S, Agrawal N, van Rossum EF, Feelders RA: Approach to the patient: diagnosis of Cushing syndrome. J Clin Endocrinol Metab. 2022, 107:3162-74. 10.1210/clinem/dgac492
- Suarez MG, Stack M, Hinojosa-Amaya JM, et al.: Hypercoagulability in Cushing syndrome, prevalence of thrombotic events: a large, single-center, retrospective study. J Endocr Soc. 2020, 4:bvz033. 10.1210/jendso/bvz033
- St-Jean M, Lim DS, Langlois F: Hypercoagulability in Cushing’s syndrome: from arterial to venous disease. Best Pract Res Clin Endocrinol Metab. 2021, 35:101496. 10.1016/j.beem.2021.101496
- Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A: Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015, 100:2807-31. 10.1210/jc.2015-1818
- Isand K, Arima H, Bertherat J, et al.: Delphi panel consensus on recommendations for thromboprophylaxis of venous thromboembolism in endogenous Cushing’s syndrome: a position statement. Eur J Endocrinol. 2025, 192:R17-27. 10.1093/ejendo/lvaf017
- McCormick JP, Sun M, Shafqat I, Heaney AP, Bergsneider M, Wang MB: Venous thromboembolic (VTE) prophylaxis in Cushing Disease patients undergoing transsphenoidal surgery. Interdiscip Neurosurg. 2022, 27:10.1016/j.inat.2021.101371
- Feelders RA, Nieman LK: Hypercoagulability in Cushing’s syndrome: incidence, pathogenesis and need for thromboprophylaxis protocols. Pituitary. 2022, 25:746-9. 10.1007/s11102-022-01261-9
- Varlamov EV, Langlois F, Vila G, Fleseriu M: Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing’s syndrome: a practical approach. Eur J Endocrinol. 2021, 184:R207-24. 10.1530/EJE-20-1309
- Fleseriu M, Biller BM, Grossman A, Swearingen B, Melmed S: Hypercoagulability in Cushing’s disease: a risk awareness and prophylaxis survey on behalf of the Pituitary Society. 15th International Pituitary Congress: Program and Abstracts. The Pituitary Society, Orlando, FL; 2017. 35.
- van Haalen FM, Kaya M, Pelsma IC, et al.: Current clinical practice for thromboprophylaxis management in patients with Cushing’s syndrome across reference centers of the European Reference Network on Rare Endocrine Conditions (Endo-ERN). Orphanet J Rare Dis. 2022, 17:178. 10.1186/s13023-022-02320-x
- Fleseriu M, Auchus R, Bancos I, et al.: Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021, 9:847-75. 10.1016/S2213-8587(21)00235-7
- Lopes V, Marques O, De Sousa Lages A: Preventive strategies for hypercoagulation in Cushing’s syndrome: when and how. Thromb J. 2023, 21:72. 10.1186/s12959-023-00515-1
- Barbot M, Daidone V, Zilio M, et al.: Perioperative thromboprophylaxis in Cushing’s disease: what we did and what we are doing?. Pituitary. 2015, 18:487-93. 10.1007/s11102-014-0600-y
- Isand K, Feelders R, Brue T, et al.: High prevalence of venous thrombotic events in Cushing’s syndrome: data from ERCUSYN and details in relation to surgery. Eur J Endocrinol. 2024, 190:75-85. 10.1093/ejendo/lvad176
- Zilio M, Mazzai L, Sartori MT, et al.: A venous thromboembolism risk assessment model for patients with Cushing’s syndrome. Endocrine. 2016, 52:322-32. 10.1007/s12020-015-0665-z
- Zaorsky NG, Buyyounouski MK, Li T, Horwitz EM: Aspirin and statin nonuse associated with early biochemical failure after prostate radiation therapy. Int J Radiat Oncol Biol Phys. 2012, 84:e13-7. 10.1016/j.ijrobp.2012.02.050
- Semple PL, Laws ER Jr: Complications in a contemporary series of patients who underwent transsphenoidal surgery for Cushing’s disease. J Neurosurg. 1999, 91:175-9. 10.3171/jns.1999.91.2.0175
- Smith TR, Hulou MM, Huang KT, Nery B, de Moura SM, Cote DJ, Laws ER: Complications after transsphenoidal surgery for patients with Cushing’s disease and silent corticotroph adenomas. Neurosurg Focus. 2015, 38:E12. 10.3171/2014.10.FOCUS14705
- Diep R, Garcia Does aspirin prevent venous thromboembolism?. Hematology Am Soc Hematol Educ Program. 2020, 2020:634-41. 10.1182/hematology.2020000150
- Maddukuri RK, Chava H, Kondaveeti ST, Mutthineni MV, Vegesana BP: Aspirin for prophylaxis of VTE in patients with hip/ knee replacement: systematic review and meta-analysis of non-randomized studies. Indian J Pharmacol. 2024, 56:420-9. 10.4103/ijp.ijp_732_21
- Spoladore R, Milani M, Spreafico LP, Agnelli G, Savonitto S: Prevention of thromboembolism after a fracture: is aspirin enough?. Eur Heart J Suppl. 2024, 26:i102-7. 10.1093/eurheartjsupp/suae025
- Undas A, Brummel-Ziedins KE, Mann KG: Antithrombotic properties of aspirin and resistance to aspirin: beyond strictly antiplatelet actions. Blood. 2007, 109:2285-92. 10.1182/blood-2006-01-010645
Filed under: Cushing's, Treatments | Tagged: Cushing's, fat, hypercortisolism, moon face, muscle wasting, muscle weakness, straie, stretch marks, thrombosis, Weight gain | Leave a comment »



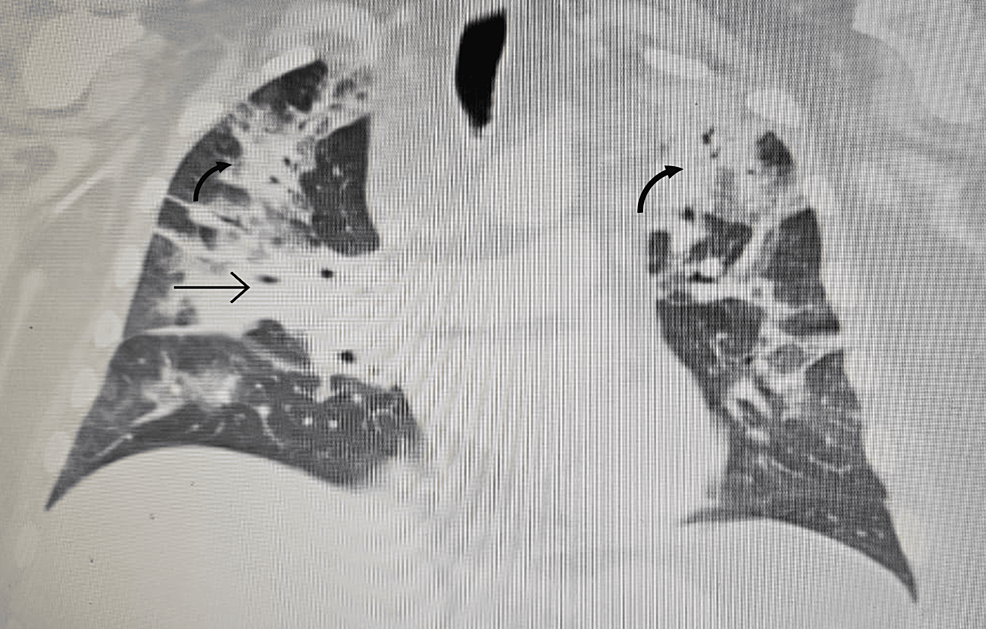
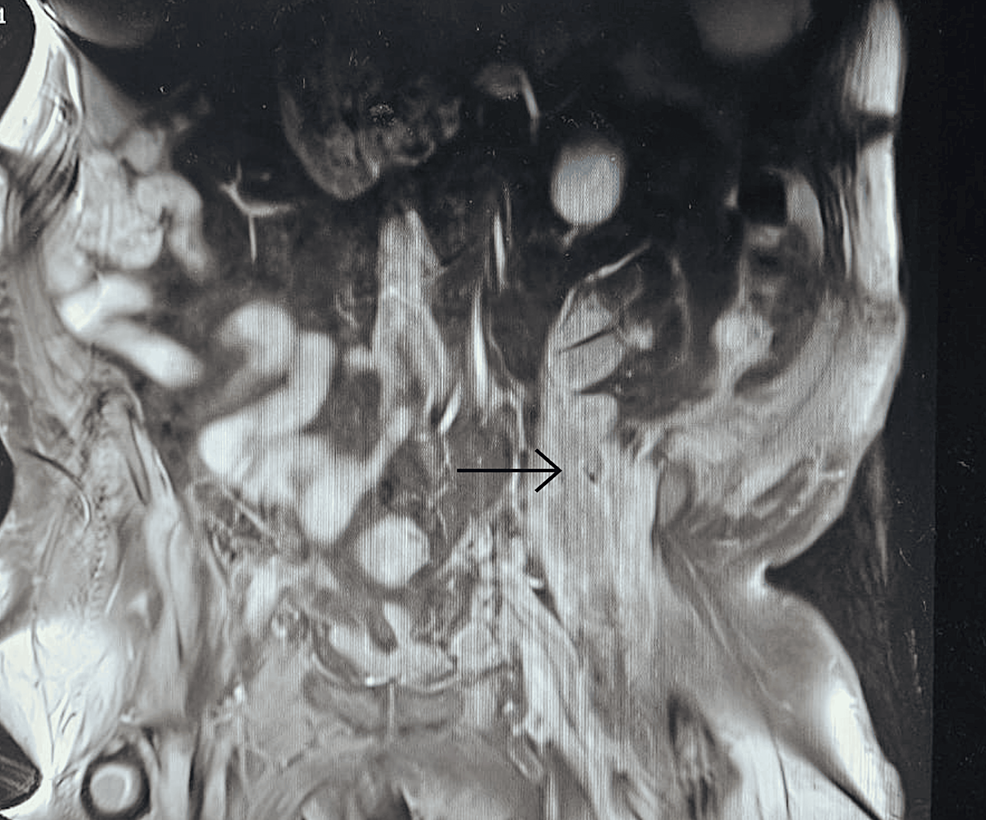
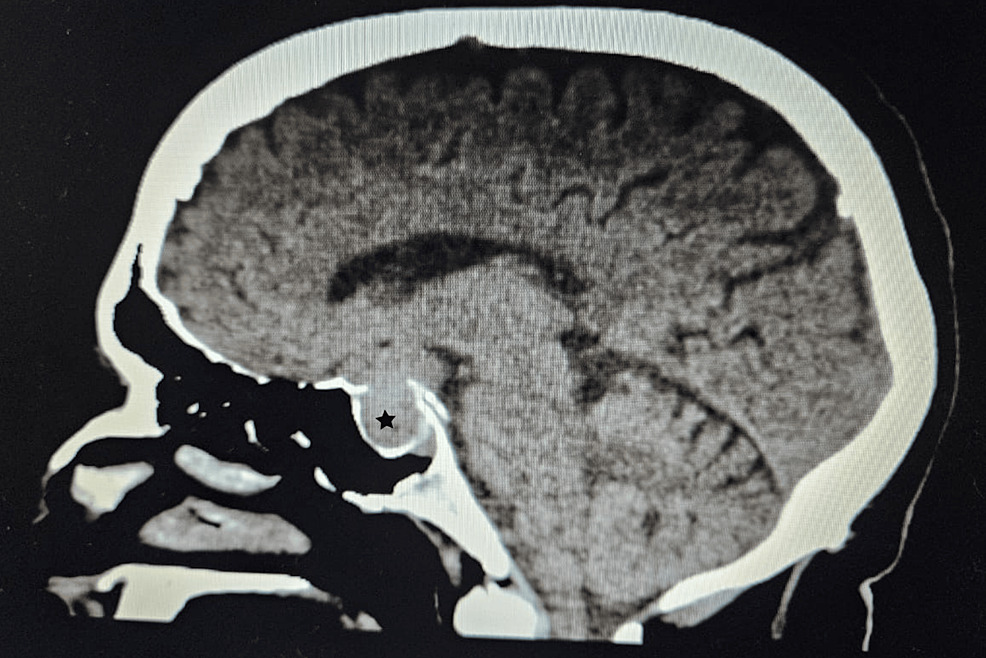
From https://www.cureus.com/articles/243881-unveiling-the-uncommon-cushings-syndrome-cs-masquerading-as-severe-hypokalemia#!/