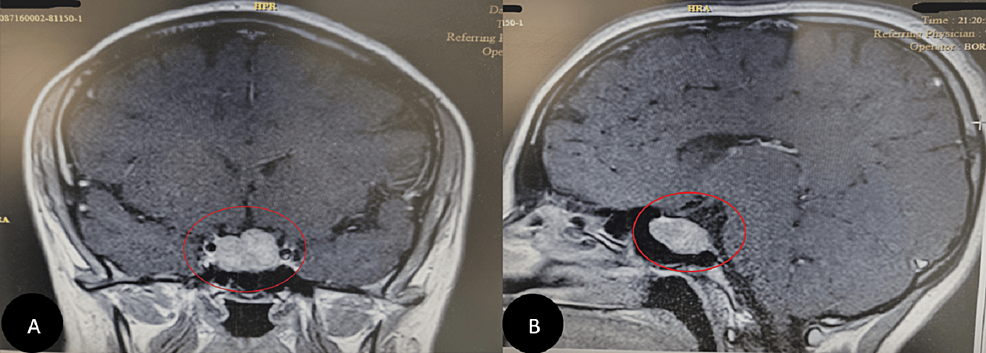
I think members of the Cushing’s Help boards have been saying this forever! Cushing’s isn’t all that rare. Just rarely diagnosed,
I think members of the Cushing’s Help boards have been saying this forever! Cushing’s isn’t all that rare. Just rarely diagnosed,
BOSTON — The prevalence of Cushing syndrome (CS) in the United States may be considerably higher than currently appreciated, new data from a single US institution suggest.
In contrast to estimates of 1 to 3 cases per million patient-years from population-based European studies, researchers at the University of Wisconsin, Milwaukee, estimated that the incidence of CS in Wisconsin is a minimum of 7.2 cases per million patient-years. What’s more, contrary to all previous studies, they found that adrenal Cushing syndrome was more common than pituitary adrenocorticotropic hormone (ACTH)– secreting tumors (Cushing disease), and that fewer than half of individuals with adrenal Cushing syndrome had classic physical features of hypercortisolism, such as weight gain, round face, excessive hair growth, and stretch marks.
“Cases are absolutely being missed…. Clinicians should realize that cortisol excess is not rare. It may not be common, but it needs to be considered in patients with any constellation of features that are seen in cortisol excess,” study investigator Ty B. Carroll, MD, Associate Professor of Medicine, Endocrinology and Molecular Medicine, and the Endocrine Fellowship Program Director at Medical College of Wisconsin in Milwaukee, told Medscape Medical News.
There are several contributing factors, he noted, “including the obesity and diabetes epidemics which make some clinical features of cortisol excess more common and less notable. Providers get used to seeing patients with some features of cortisol excess and don’t think to screen. The consequence of this is more difficult-to-control diabetes and hypertension, more advance metabolic bone disease, and likely more advanced cardiovascular disease, all resulting from extended exposure to cortisol excess,” he said.
Are Milder Cases the Ones Being Missed?
Asked to comment, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University College of Physicians and Surgeons, New York City, said “When we talk about Cushing [syndrome], we usually think of pituitary ACTH as more [common], followed by adrenal adenomas, and then ectopic. But they’re seeing more adrenal adenoma…we are probably diagnosing this a little more now.”
She also suggested that the Wisconsin group may have a lower threshold for diagnosing the milder cortisol elevation seen with adrenal Cushing syndrome. “If you screen for Cushing with a dexamethasone suppression test…[i]f you have autonomous secretion by the adrenal, you don’t suppress as much…. When you measure 24-hour urinary cortisol, it may be normal. So you’re in this in-between [state]…. Maybe in Wisconsin they’re diagnosing it more. Or, maybe it’s just being underdiagnosed in other places.”
She also pointed out that “you can’t diagnose it unless you think of it. I’m not so sure that with these mild cases it’s so much that it’s more common, but maybe it’s like thyroid nodules, where we didn’t know about it until everybody started getting all of these CT scans. We’re now seeing all these incidental thyroid nodules…I don’t think we’re missing florid Cushing.”
However, Wardlaw said, it’s probably worthwhile to detect even milder hypercortisolism because it could still have long-term damaging effects, including osteoporosis, muscle weakness, glucose intolerance, and frailty. “You could do something about it and normalize it if you found it. I think that would be the reason to do it.”
Is Wisconsin Representative of Cushing Everywhere?
Carroll presented the findings at the annual meeting of the Endocrine Society. He began by noting that most of the previous CS incidence studies, with estimates of 1.2-3.2 cases per million per year, come from European data published from 1994 to 2019 and collected as far back as 1955. The method of acquisition of patients and the definitions of confirmed cases varied widely in those studies, which reported CS etiologies of ACTH-secreting neoplasms (pituitary or ectopic) in 75%-85% and adrenal-dependent cortisol excess in 15%-20%.
The current study included data from clinic records between May 1, 2017, and December 31, 2022, of Wisconsin residents newly diagnosed with and treated for CS. The CS diagnosis was established with standard guideline-supported biochemical testing and appropriate imaging. Patients with exogenous and non-neoplastic hypercortisolism and those who did not receive therapy for CS were excluded.
A total of 185 patients (73% female, 27% male) were identified from 27 of the total 72 counties in Wisconsin, representing a population of 4.5 million. On the basis of the total 5.9 million population of Wisconsin, the incidence of CS in the state works out to 7.2 cases per million population per year, Carroll said.
However, data from the Wisconsin Hospital Association show that the University of Wisconsin’s Milwaukee facility treated just about half of patients in the state who are discharged from the hospital with a diagnosis of CS during 2019-2023. “So…that means that an actual or approximate incidence of 14-15 cases per million per year rather than the 7.2 cases that we produce,” he said.
Etiologies were 60% adrenal (111 patients), 36.8% pituitary (68 patients), and 3.2% ectopic (6 patients). Those proportions were similar between genders.
On biochemical testing, values for late-night salivary cortisol, dexamethasone suppression, and urinary free cortisol were highest for the ectopic group (3.189 µg/dL, 42.5 µg/dL, and 1514.2 µg/24 h, respectively) and lowest for the adrenal group (0.236 µg/dL, 6.5 µg/dL, and 64.2 µg/24 h, respectively). All differences between groups were highly statistically significant, at P < .0001, Carroll noted.
Classic physical features of CS were present in 91% of people with pituitary CS and 100% of those ectopic CS but just 44% of individuals with adrenal CS. “We found that adrenal-dependent disease was the most common form of Cushing syndrome. It frequently presented without classic physical features that may be due to the milder biochemical presentation,” he concluded.
Carroll reports consulting and investigator fees from Corcept Therapeutics. Wardlaw has no disclosures.
Miriam E. Tucker is a freelance journalist based in the Washington DC area. She is a regular contributor to Medscape, with other work appearing in The Washington Post, NPR’s Shots blog, and Diatribe. She is on X (formerly Twitter) @MiriamETucker.
Lead image: Designer491/Dreamstime
Medscape Medical News © 2024 WebMD, LLCSend comments and news tips to news@medscape.net.
Filed under: adrenal, Cushing's, pituitary, Rare Diseases, symptoms | Tagged: ACTH, adrenal, Cushing's Syndrome, hair, MaryO, pituitary, rare, round face, stretch marks, Weight gain | Leave a comment »