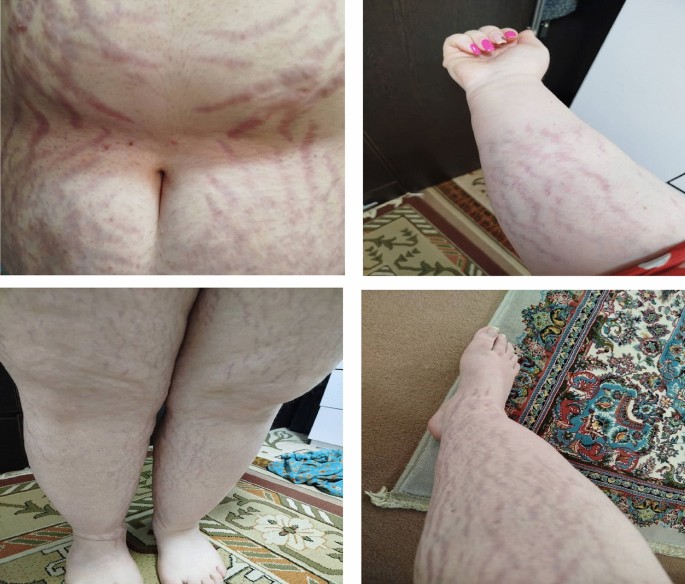
Myth: “Each person requires the same dose of steroid in order to survive with Secondary or Primary Adrenal Insufficiency”
Fact: In simple terms, Adrenal Insufficiency occurs when the body does not have enough cortisol in it. You see, cortisol is life sustaining and we actually do need cortisol to survive. You have probably seen the commercials about “getting rid of extra belly fat” by lowering your cortisol. These advertisements make it hard for people to actually understand the importance of the function of cortisol.
After a Cushing’s patient has surgery, he/she goes from having very high levels of cortisol to no cortisol at all. For pituitary patients, the pituitary, in theory, should start working eventually again and cause the adrenal glands to produce enough cortisol. However, in many cases; the pituitary gland does not resume normal functioning and leaves a person adrenally insufficient. The first year after pit surgery is spent trying to get that hormone to regulate on its own normally again. For a patient who has had a Bilateral Adrenalectomy (BLA), where both adrenal glands are removed as a last resort to “cure” Cushing’s; his/her body will not produce cortisol at all for his/her life. This causes Primary Adrenal Insufficiency.
All Cushing’s patients spend time after surgery adjusting medications and weaning slowly from steroid (cortisol) to get the body to a maintenance dose, which is the dose that a “normal” body produces. This process can be a very long one. Once on maintenance, a patient’s job is not over. He/She has to learn what situations require even more cortisol. You see, cortisol is the stress hormone and also known as the Fight or Flight hormone. Its function is to help a person respond effectively to stress and cortisol helps the body compensate for both physical and emotional stress. So, when faced with a stressor, the body will produce 10X the baseline levels in order to compensate. When a person can not produce adequate amounts of cortisol to compensate, we call that Adrenal Insufficiency. If it gets to the point of an “Adrenal Crisis”, this means that the body can no longer deal and will go into shock unless introduced to extremely high levels of cortisol, usually administered through an emergency shot of steroid.
There are ways to help prevent a crisis, by taking more steroid than the maintenance dose during times of stress. This can be anything from going to a family function (good stress counts too) to fighting an infection or illness. Acute stressors such as getting into a car accident or sometimes even having a really bad fight require more cortisol as well.
It was once believed that everyone responded to every stressor in the exact same way. So, there are general guidelines about how much more cortisol to introduce to the body during certain stressors. For instance, during infection, a patient should take 2-3X the maintenance dose of steroid (cortisol). Also, even the maintenance dose was considered the same for everyone. Now a days, most doctors will say that 20 mg of Hydrocortisone (Steroid/Cortisol) is the appropriate maintenance dose for EVERYONE. Now, we know that neither is necessarily true. Although the required maintenance dose is about the same for everyone; some patients require less and some require more. I have friends who will go into an adrenal crisis if they take LESS than 30 mg of daily steroid. On the other hand, 30 mg may be way too much for some and those folks may even require LESS daily steroid, like 15 mg. Also, I want to stress (no pun intended) that different stressors affect different people differently. For some, for instance, an acute scare may not affect them. However, for others, receiving bad news or being in shock WILL put their bodies into crisis. That person must then figure out how much additional steroid is needed.
Each situation is different and each time may be different. Depending on the stressor, a person may need just a little more cortisol or a lot. Every person must, therefore, learn their own bodies when dealing with Adrenal Insufficiency. This is VERY important! I learned this the hard way. As a Clinical Psychologist; I assumed that my “coping skills” would be enough to prevent a stressor from putting me into crisis. That was FAR from the truth! I have learned that I can not necessarily prevent my body’s physiological response to stress. People often ask me, “BUT you are a psychologist! Shouldn’t you be able to deal with stress?!!!!” What they don’t realize is that my BODY is the one that has to do the job of compensating. Since my body can not produce cortisol at all, my job is to pay close attention to it so that I can take enough steroid to respond to any given situation. We all have to do that. We all have to learn our own bodies. This is vitally important and will save our lives!
To those we have lost in our community to Adrenal Insufficiency after treatment of Cushing’s, Rest in Peace my friends! Your legacies live on forever!
~ By Karen Ternier Thames
Filed under: adrenal, adrenal crisis, Cushing's, Myths and Facts, pituitary, Treatments | Tagged: adrenal crisis, adrenal glands, adrenal insufficiency, bilateral laparoscopic adrenalectomy, BLA, cortisol, Cushing's, hydrocortisone, In memory, Karen Ternier Thames, Myths and Facts, Pituitary gland, steroid, steroids, surgery | Leave a comment »