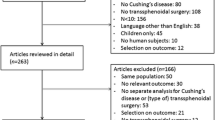
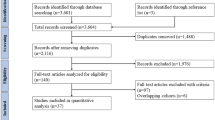
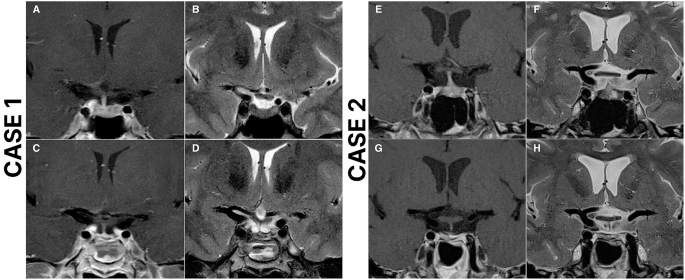
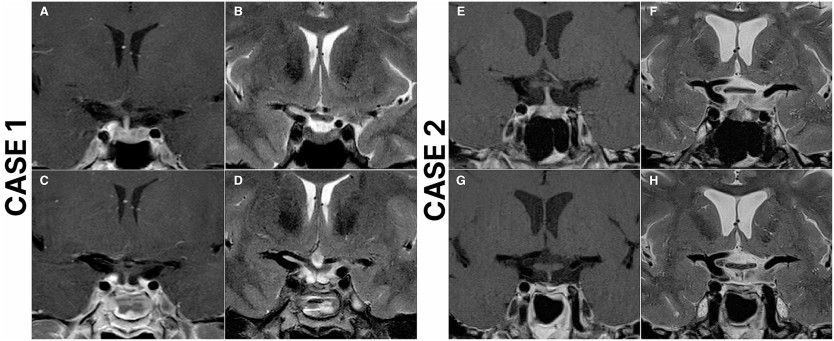
Introduction
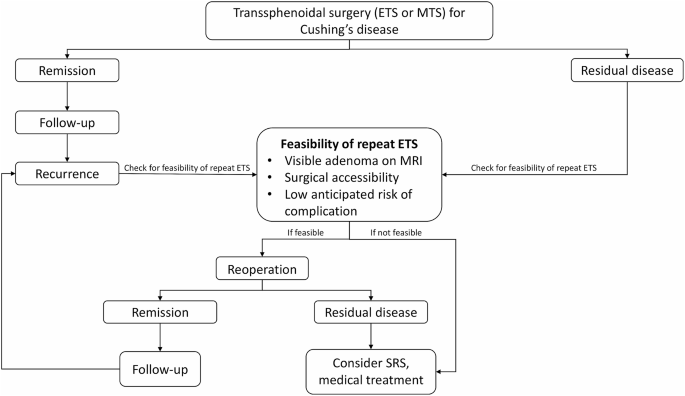
Methods
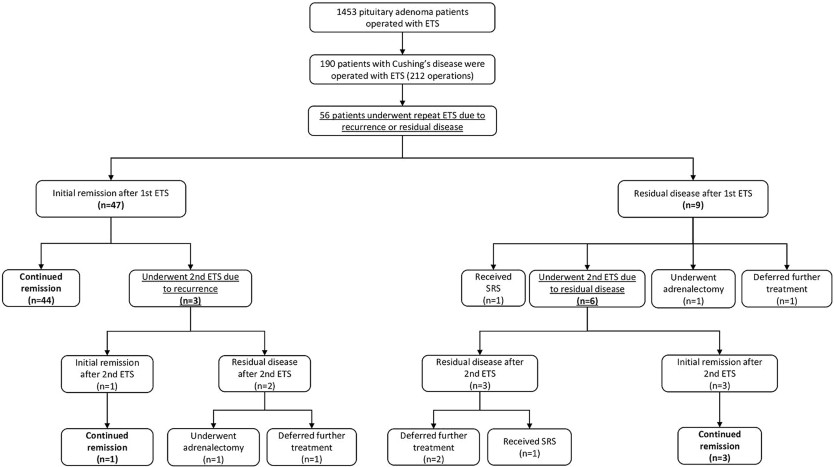
Results
Conclusion
Key words
Abbreviations
Introduction
Methods
Study Population
Preoperative Investigations
Surgical Procedure
Postoperative Investigations and Follow-up
Data Collection
Statistical Analysis
Ethics Statement
Results
Baseline Characteristics
Table 1. Baseline Characteristics of the Study Population
| Number of Patients | 125 |
|---|---|
| Patient variables | |
| Age | Median 48 (range: 14–79) years |
| Male | 32 (25.6%) |
| Pituitary characteristics | |
| Microadenoma | 57 (45.6%) |
| Macroadenoma | 41 (32.8%) |
| No focal lesion on pituitary MRI (MRI-negative Cushing’ disease) | 26 (20.8%) |
| Operative variables | |
| Single operation | 88 (70.4%) |
| More than 1 operation | 33 (26.4%) |
| 3 operations | 4 (3.2%) |
Perioperative Complications
Table 2. Procedural Complications Encountered in Our Series
| Complication | N (%) |
|---|---|
| Any complication | 40 (32.0%) |
| Persistent diabetes insipidus | 18 (14.4%) |
| Cerebrospinal fluid leak | 17 (13.6%) |
| Meningitis | 2 (1.6%) |
| Ventriculitis | 1 (0.8%) |
| Bleeding/haematoma | 4 (3.2%) |
| Visual deterioration | 2 (1.6%) |
| Death | 3 (2.4%) |
Disease Remission
Table 3. Multivariable Logistic Regression for Predictors of Remission at Last Follow-up
| Outcome | Predictor | Multivariable Analysis | |
|---|---|---|---|
| Adjusted OR (95% CI) | P Value | ||
| Remission at 3 months | Age | 1.04 (95%CI: 1.01–1.07) | 0.009 |
| Sex† | 3.31 (95%CI: 1.31–8.40) | 0.011 | |
| MRI-negative Cushing disease‡ | 1.88 (95%CI: 1.06–3.35) | 0.031 | |
| Single operation§ | 3.87 (95%CI: 1.56–9.61) | 0.004 | |
| Remission at last follow-up | Age | 1.00 (95%CI: 0.97–1.04) | 0.904 |
| Sex† | 2.92 (95%CI: 0.89–9.62) | 0.003 | |
| MRI-negative Cushing disease‡ | 1.84 (95%CI: 0.89–3.79) | 0.008 | |
| Single operation§ | 1.24 (95%CI: 0.36–4.24) | 0.730 | |
| Remission at 3 months∗ | 27.0 (95%CI: 8.47–83.33) | <0.001 | |
- ∗
-
No remission is used as the reference group.
- †
-
Female is used as the reference group.
- ‡
-
Microadenoma is used as the reference group.
- §
-
Multiple operation is used as the reference group.
Growth Hormone Replacement Therapy
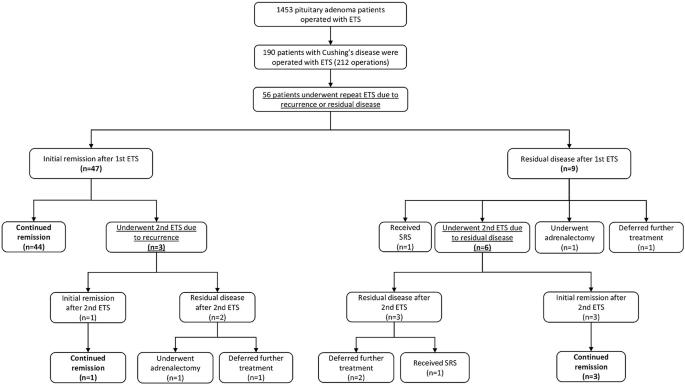
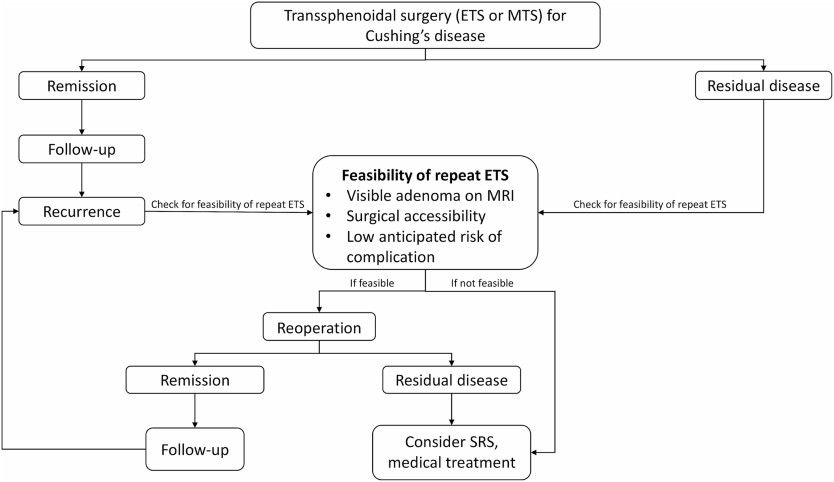
Secondary Intervention
DISCUSSION
Remission
Complications
Limitations
Conclusions
CRediT authorship contribution statement
-
Cushing disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literatureJ Clin Endocrinol Metab, 96 (2011), pp. 632-642
- 2
J. Etxabe, J.A. VazquezMorbidity and mortality in Cushing disease: an epidemiological approachClin Endocrinol (Oxf), 40 (1994), pp. 479-484
- 3
O. Ragnarsson, D.S. Olsson, D. Chantzichristos, et al.The incidence of Cushing disease: a nationwide Swedish studyPituitary, 22 (2019), pp. 179-186
- 4
M. Boscaro, G. ArnaldiApproach to the patient with possible cushing’s syndromeJ Clin Endocrinol Metab, 94 (2009), pp. 3121-3131
- 5
C.M. Plotz, A.I. Knowlton, C. RaganThe natural history of cushing’s syndromeAm J Med, 13 (1952), pp. 597-614
- 6
A.B. Atkinson, A. Kennedy, M.I. Wiggam, D.R. McCance, B. SheridanLong-term remission rates after pituitary surgery for Cushing disease: the need for long-term surveillanceClin Endocrinol (Oxf), 63 (2005), pp. 549-559
- 7
L.H.A. Broersen, N.R. Biermasz, W.R. van Furth, et al.Endoscopic vs. microscopic transsphenoidal surgery for Cushing disease: a systematic review and meta-analysisPituitary, 21 (2018), pp. 524-534
- 8
R. Pivonello, M. De Leo, A. Cozzolino, A. ColaoThe treatment of cushing’s diseaseEndocr Rev, 36 (2015), pp. 385-486
- 9
H.-D. Jho, R.L. CarrauEndoscopic endonasal transsphenoidal surgery: experience with 50 patientsJ Neurosurg, 87 (1997), pp. 44-51
- 10
P. Cappabianca, A. Alfieri, E. de DivitiisEndoscopic endonasal transsphenoidal approach to the sella: towards Functional Endoscopic Pituitary Surgery (FEPS)min – Minim Invasive Neurosurg, 41 (1998), pp. 66-73
- 11
J. Jagannathan, R. Smith, H.L. DeVroom, et al.Outcome of using the histological pseudocapsule as a surgical capsule in Cushing diseaseJ Neurosurg, 111 (2009), pp. 531-539
- 12
M. Mayberg, S. Reintjes, A. Patel, et al.Dynamics of postoperative serum cortisol after transsphenoidal surgery for Cushing disease: implications for immediate reoperation and remissionJ Neurosurg, 129 (2018), pp. 1268-1277
- 13
R.A. Alwani, W.W. de Herder, M.O. van Aken, et al.Biochemical predictors of outcome of pituitary surgery for Cushing diseaseNeuroendocrinology, 91 (2010), pp. 169-178
- 14
E. Valassi, H. Franz, T. Brue, et al.Preoperative medical treatment in Cushing syndrome: frequency of use and its impact on postoperative assessment: data from ERCUSYNEur J Endocrinol, 178 (2018), pp. 399-409
- 15
L.B. Yap, H.E. Turner, C.B.T. Adams, J.A.H. WassUndetectable postoperative cortisol does not always predict long-term remission in Cushing disease: a single centre auditClin Endocrinol (Oxf), 56 (2002), pp. 25-31
- 16
P. Cappabianca, L.M. Cavallo, A. Colao, E. de DivitiisSurgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomasJ Neurosurg, 97 (2002), pp. 293-298
- 17
P. Leach, A.H. Abou-Zeid, T. Kearney, J. Davis, P.J. Trainer, K.K. GnanalinghamEndoscopic transsphenoidal pituitary surgery: evidence of an operative learning curveNeurosurgery, 67 (2010), pp. 1205-1212
- 18
C. Snyderman, A. Kassam, R. Carrau, A. Mintz, P. Gardner, D.M. PrevedelloAcquisition of surgical skills for endonasal skull base surgery: a training ProgramLaryngoscope, 117 (2007), pp. 699-705
- 19
P.C. Johnston, L. Kennedy, A.H. Hamrahian, et al.Surgical outcomes in patients with Cushing disease: the Cleveland clinic experiencePituitary, 20 (2017), pp. 430-440
curation, Writing – review & editing. Eleni Maratos: Data curation, Writing – review & editing. Sinan Barazi: Data curation, Writing – review & editing. Simon Aylwin: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Nick WM. Thomas: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing.
References
- 1
R.N. Clayton, D. Raskauskiene, R.C. Reulen, P.W. JonesMortality and morbidity in
Filed under: Cushing's, pituitary, Treatments | Tagged: Cushing's Disease, endoscopic, pituitary, remission, transsphenoidal | Leave a comment »